Sleep, gut dysbiosis, Sulfur metabolism, Melatonin, SIBO, chimeric spike & cancer risk.
Autoimmune thyroiditis & fetal risks...and toss in a conversation with Dr. Hazan, a sulfur protocol by Chris Masterjohn and some zinc and resistant starch to feed a healthier gut microbiome.
Dear readers, I’ve been really busy with a complex research direction, and it helps me to not change focus, so here is an draft post that is disorganized but has some valuable topics and research/resources. Happy Saturday to you. My apologies for being distracted lately as a blogger. Beyond CoV and personal health topics, I have lost the Thread of what my subscribers are interested in, so this is somewhat CoV and personal health related.
Summary of a few take-home points
1. Regular sleep cycle (aiming for within 90 minutes of a routine sleep and wake time) may help promote a healthy gut microbiome and it may make some melatonin and help reduce inflammation for us too.
2. Excess dopamine levels prenatally, or hyperthyroidism may increase the infant’s risk for epilepsy or schizophrenia.
3. Vegans may be at increased risk for SIBO (Small Intestine Bacterial Overgrowth) because they are eating a lot more resistant starch, which feeds good species, but it might be overgrown because of an imbalance in sulfur metabolism within the colon. The colon needs to be anaerobic, low oxygen, in order to promote the correct balance of colon microbes, that don’t ‘swim upstream’ where there is oxygen. We need the small intestine microbiome and colon microbiome to be more ‘seperate but equally important’.
4. In order to have a healthy microbiome, avoid modern processed food and eat a generous amount of raw produce and cooked/chilled resistant starches (like tapioca beads in ‘Bubble Tea’) and have a generous amount of zinc in the diet. Beneficial species need about 30% of the zinc we eat each day, while negative species can survive on processed food and lack of important nutrients.
This older post was ‘Liked’ recently, and it is a good post, somewhat related - lack of antioxidants and selenium, or lack of iodine or excess fluoride/bromide and perchlorate may increase risk of autoimmune thyroiditis which can have hyper or hypothyroid symptoms, and neither would be good for a developing fetus (one of the many subpoints mixed into the later parts of this post).
And this post has more about autoimmune thyroiditis (selenium! antioxidants! Nrf2 promoting phytonutrients and/or omega 3 fatty acids!)
~~
Regarding the following conversation with Dr. Hazan, I haven’t followed up yet on any research with her. I have been working on something else in an intensive way. I’ve shared a little of that here but it may not be topics CoV/personal health readers are currently interested in, so hopefully, this will be of more interest.
“The Art of Medicine starts with asking the right questions. The Art of Science starts with making the right hypothesis… Happy Sunday…” - Sabine Hazan, M.D. (x.com/SabinehazanMD
My reply:
“What percent of our dietary zinc is consumed by beneficial butyrate producing gut microbiome species like Bifidobacterium? Answer ~ 30%
How much resistant starch/fiber do they need us to eat each day? a capsule? a teaspoon? a 1/2 cup of a resistant starch food? fresh produce?
Answer ~ quite a bit, fresh produce and the 1/2 cup of a resistant starch food. .... not just a capsule or teaspoon.” (x.com/deNutrients)
Which didn’t go over as well as I hoped: “Did you ask @grok that? “SHOW ME THE DATA”” - Sabine Hazan, MD
My reply/s:
This is my area of research. One line from my ~ 100 page unpublished paper:
"Zinc is needed for butyrate producing microbiome species to thrive. (Tako, Koren, 2020)" https://sciforum.net/paper/view/6993
Regarding how much resistant starch should be in the diet ... a whopping 5-15% of total daily calories: “The overall diet goal roughly is 5-15% resistant starch foods." Excerpt from my webpage RS/Butyrate-How much? https://jenniferdepew.com/rs%2Fbutyrate-how-much%3F…
*I am not the enemy, I've mentioned that before. There is value in a multi-disciplinary team because you may not see what you aren't aware of.
That is a large amount, not a capsule or teaspoon but a teaspoon of raw starch would have a lot more than after cooking.
"113 mg in a serving of rice that was cooked and then chilled, providing about 3% resistant starch. One to three percent may be average for resistant starch foods once cooked, so it seems many portions throughout the day are needed to reach 5-15% [of the total daily intake], or include some raw sources too, green banana flour smoothies?"
Good sources of resistant starch have 20-30% of the starches as resistant starch. Cooking may bring that down to 2-3%. Cooked rice after chilling has 3% resistant starch, ½ cup serving would provide 103 calories, 23 grams of starch - with approximately 3% as resistant starch, and potentially some butyrate and other SCFAs, three calories worth - is less than a gram of carbohydrate, 750 milligrams potential SCFAs, “∼60% acetate, 25% propionate, and 15% butyrate, ” (123) - equals 113 milligrams butyrate, 187 milligrams propionate, and 450 milligrams acetate. (128)"
Eating a teaspoon of raw starch might increase the amount of resistant starch available to the gut, in fewer calories.
"Canna and yam flour contained similar amounts of amylose (32.7 % and 33.1 %, respectively), followed by arrowroot (29.4 %), sweet potato (26.8 %) and konjac (21.7 %). Taro and cassava had the lowest amounts of amylose of all with 17.3 % and 13.1 %, respectively. Among the starch samples, canna contained the highest amount of amylose (35.0 %) followed by similar amounts for yam (23.7 %), arrowroot (21.9 %) and konjac (21.3 %). Sweet potato and cassava also contained comparable concentrations of amylose (15.9 % and 14.6 %, respectively) while taro was the lowest with only 10.1 %." (8)"
"Naturally, different types of rice will have varying levels of amylose (resistant starch) content ranging from 0-35%. Digestibility of cooked rice starch is typically influenced by the amount of amylose, with higher amylose content resulting in slower digestion and lower glycemic index of the rice. However, after cooking rice, there is typically less than 3% resistant starch that will not get digested. " (26)
(Incomplete) reference list: https://docs.google.com/document/d/1Mb7ltLGHh2MDuc3u3bcr0MDhlyl4cs-MK0yGMs1Vwv8/edit?usp=sharing
Reply 2: An important question to ask in the medical treatment of gut dysbiosis, is "What do beneficial species need to eat, and how much?" = lots of zinc and resistant starch/fiber
and another is "What can negative gut species eat that beneficial species can't survive on?" = Ultra-processed foods and a low mineral diet.
As a dietitian who worked as a prenatal counselor, I tend to look at research with the mental questions of "How is this protein or tissue type formed by the body?" and "What nutrients are needed to form it or are needed as enzyme cofactors?" We can't grow a baby or a new cell without nutrients and enzymes and DNA transcription factors.
The gut microbiome is a garden which we need to tend with balanced fertilizer and hydration.
She responded to a separate reply: “*If this was new information to you, then why would you expect Grok to know it? Please respect that I have put in massive numbers of hours to give you that brief summary. Thank you and Happy Sunday to you Dr. Hazan. I greatly admire your tenacity and work.”
“Then put those hours to write the data. Its not easy. Perhaps I can help you in 2025. More time on my hands. Make a zoom meeting with me by reaching @Progenabiome Let's show the data…” x.com/SabinehazanMD”
and a second: “Of course, I myself have to fight those who retract papers but I think you can handle being on the battlefield.”
Well thanks Sabine!
Melatonin is made in the pineal gland while we sleep, but lots more of it is produced within the gut by beneficial species. SARS-CoV-2/chimeric spike seems to knock out the beneficial gut species (Bifidobacterium in particular, research by Sabine Hazan) and lead to low melatonin levels in the body and a greatly increased risk for SIBO. Small Intestine Bacterial Overgrowth, is really a displacement of colon species into the small intestines or vice versa. Bifidobacterium longum is a species that has been associated with better sleep quality. (Patterson, et al., 2024)
Changes in sleep timing of as little as 90 minutes difference in the average midpoint can negatively affect the gut microbiome species towards dysbiosis and decrease mental efficiency for the person. The impact of sleep changes on daily function is called “social jet lag” in the article. (sciencedaily.com)
The following article doesn’t mention melatonin or suggest that Bifidobacterium longum are making melatonin…. however an increase in melatonin in the gut could help explain the benefits that were observed.
Melatonin is an inhibitor of TNF-alpha: “…melatonin has been observed to inhibit TNF-α production in the pineal gland, where high levels of TNF-α correlate with the inhibition of nocturnal melatonin synthesis.” (Brave AI summary; Clark and Vissel, 2014; Huang, et al., 2019)
“In brief, therefore, we note that melatonin is well-recognized as an inhibitor of TNF [35–37], and that TNF, in turn, transiently inhibits its production [38].” (Clark and Vissel, 2014)
So, the finding that chimeric spike is inhibiting Nrf2 (the ankyrin repeat domain is likely involved) and promoting TNF-alpha, would help explain why less melatonin production is being seen post CoV infection or injection. TNF-alpha inhibits melatonin and melatonin adequacy would help inhibit TNF-alpha.
Niacin, high dose, or pomegranate peel or other Nrf2 promoters would help as they also inhibit NF-kB and TNF-alpha. Nrf2 shares a Clock protein with NF-kB, so the two pathways can’t really function at the same time. They seem to be circadian cycle proteins which modern life tips towards remaining in the daytime mode of increased TNF-alpha. TNF-alpha is involved in sleep and routinely increases towards the end of the day, peaking before sleep onset. Orexin is also involved. (Clark and Vissel, 2014)
“As recently summarized [33], wakefulness enhances TNF protein levels and expression in brain, and the highest normal brain levels, at least in the rat, occur at the time of usual sleep onset. Sleep deprivation elevates levels even further, the effects of which we experience in jetlag.” (Clark and Vissel, 2014)
“The mechanisms through which probiotics and other gut microbiome modulators may influence sleep health via the microbiome-gut-brain axis have recently been described35. Microbial metabolites within the gut, the serotonergic system, the vagus nerve, and peripheral immune reactions can all regulate sleep via the microbiome-gut-brain axis35. Furthermore, the relationship between stress, sleep, and the immune system is well established. The hypothalamic pituitary adrenal (HPA) axis, autonomic nervous system, and the enteric nervous system all directly interact with the immune system13,22. Stress is associated with increased inflammation and as such, chronic low-grade inflammation is a biological driver of adverse health outcomes associated with stress88. Stress can also have an impact on the environment of the gut and the composition of the gut microbiota, while probiotic administration can influence the stress response13. Sleep is also considered an important modulator of the immune system, whereby disrupted sleep is associated with a pro-inflammatory state89,90. Viral and bacterial infections can also have a negative impact on sleep health91. Taken together, this suggests a bidirectional route of communication via the microbiome-gut-brain axis, which supports the close relationship between stress, sleep, and immunity. The immunoregulatory effects of probiotics have been proposed to occur through the generation of T regulatory cell populations and the synthesis and secretion of the anti-inflammatory cytokine, IL-1012,92. In a recent study in females with IBS, B. longum 1714 in combination with B. longum 35624 improved anxiety, depression, and sleep quality which was associated with a decrease in the pro-inflammatory cytokine TNF-α15. Thus, it is plausible that the positive effects of B. longum 1714 on stress and sleep occur through immunoregulation within the peripheral immune system and that these signals are communicated to the brain either directly or indirectly via the microbiome-gut-brain axis. Furthermore, the microbial production of tryptophan which can in turn increase serotonin availability in both the peripheral and central nervous system93 can promote healthy sleep93,94,95, as serotonin can be converted to melatonin, the key molecule with hormonal properties that regulates sleep/wake cycles96. It was previously shown that 6 weeks administration of a probiotic mixture containing B. longum increased salivary melatonin levels97. Considering the proposed mechanisms through which B. longum 1714 can have a positive effect on stress, sleep, and energy, future studies investigating the effect of this strain on overall mental wellness should focus on underpinning the specific mechanism of action to determine whether our hypotheses can be supported.
Overall, this study has demonstrated that the B. longum 1714 strain, which has previously demonstrated promising effects on stress, may also benefit well-being and sleep. B. longum 1714 was well tolerated and improved both sleep quality and daytime dysfunction after 4 weeks and improved energy/vitality and social functioning after 8 weeks. Considering both sleep and stress are highly placebo-responsive, these results in a healthy population are highly encouraging and call for additional investigation to better understand the capacity of B. longum 1714 to improve sleep and other mental wellness-related health indications. The development of novel interventions to improve sleep health, and particularly probiotics that positively affect the gut microbiome without negatively impacting other functions, will contribute to the overall well-being of individuals, because sleep is a fundamental measure of health.” (Patterson, et al., 2024)
The small intestine is supposed to be aerobic - oxygen using species while the colon is supposed to be an anaerobic environment, using resistant starch for energy and producing butyrate and other short chain fatty acids which the colon cells use as an anaerobic energy source. When that is thrown off, and the colon is aerobic, then cancer is more likely. Butyrate also signals for immune functions to occur which would protect against cancer.
“Quinine consumption has been shown to reduce appetite and food intake in human and mice. Here, tested on two common mouse strains, C3H/lbg and C57BL/6J, it exerted a different effect. While quinine reduced weight gain in C3H/lbg mice, C57BL/6J were unaffected by the bitter molecule. Among the differences between the two strains, C57BL/6J present a blunted Melatonin production. In this study, we investigate if endogenous Melatonin is playing any role in the different response of C57BL/6J mice to quinine. The effect of dietary supplementation with Melatonin as well as of endogenous gastrointestinal and pineal produced Melatonin was investigated by supplementing quinine diet with pure Melatonin, L-Tryptophan or by reversion of light/dark cycle, respectively. The consumption of Melatonin reverts the phenotype and makes C57BL/6J mice sensitive to quinine. Similarly, quinine potency in C3H/ Ibg mice augments upon supplementation with exogenous Melatonin or upon increase of Melatonin endogenous levels. In vivo, as well as in in vitro cell cultures, Melatonin Receptor modulation inhibits quinine dependent secretion of Ghrelin, while potentiates quinine dependent secretion of Cholecystokinin. Acting via Melatonin Receptors, Melatonin tunes the effect of quinine, reducing and potentiating its effect in enteroendocrine cells of the upper and the lower digestive tract, respectively. Our results indicate that signaling pathways activated by Melatonin tunes the activity of Bitter Receptors located in the gastrointestinal tract.”
What other gut species are associated with better sleep?
Pomegranate peel would help promote Bacteroidetes in balance with Firmicutes and therefore may be helping us with abstract thinking! (Smith, et al., 2019)
“We found that total microbiome diversity was positively correlated with increased sleep efficiency and total sleep time, and was negatively correlated with wake after sleep onset. We found positive correlations between total microbiome diversity and interleukin-6, a cytokine previously noted for its effects on sleep. Analysis of microbiome composition revealed that within phyla richness of Bacteroidetes and Firmicutes were positively correlated with sleep efficiency, interleukin-6 concentrations and abstract thinking. Finally, we found that several taxa (Lachnospiraceae, Corynebacterium, and Blautia) were negatively correlated with sleep measures.” (Smith, et al., 2019)
Vegetarian and Vegan Diets: A diet high in vegetables, fruits, and whole grains can positively correlate with the abundance of Lachnospiraceae and Blautia. These diets provide a rich source of fiber and other plant-based nutrients that these bacteria can metabolize. (Brave AI summary)
Brave AI assures us that anyone can get SIBO, not just vegans and not all vegans have SIBO. (summary) Someone on Quora asked “Why do Vegans get SIBO?” and the answer is the high fiber content feeds the SIBO species, but I think there is more to it than that. (Quora) Problems in sulfation metabolism can upset the colon anaerobic environment and be causal in SIBO. See this post, or paywalled posts by Chris Masterjohn, PhD, (one), My Sulfur Protocol, (two). Sulfur metabolism is involved in keeping the colon anaerobic, but I would need to reread it.
Chris’s work gets deep but he is ahead of the curve on accuracy and worth the paid subscription in my personal opinion.
“Do you ever have anxiety? Depression?
Tremors, twitches, heart palpitations or spasms?
Any psychiatric or neurological issue can be a sulfur problem.
If you care about your mitochondrial health and your longevity, you need to care about your sulfur metabolism.
There are different types of sulfur molecules that can accumulate to toxic levels, so the problems aren’t always the same.
For one person, it’s fatigue and nausea. For another, it’s diarrhea or poor digestion. For yet another, it’s strange purple spots on their skin.
For another, it’s negative reactions to supplements like thiamin, glutathione, or B6.” - ‘My Sulfur Protocol’, - Chris Masterjohn, (two).
I did experience B6 sensitivity recently - I was getting finger numbness symptoms as if deficient in vitamin B12, but then I learned that it is also a symptom of excess B6 and the finger numbness/tingling got better when I stopped taking high dose B6 along with my other high dose B vitamins. I try to provide helpful information, but physiology is very complex and I am still learning too. *I do seem to need some extra though. I’ve been taking ~ 50 mg daily or when I remember instead of 250 mg plus 100 mg later in the day (when remembered, I am inconsistent with self-care, which is not ideal.)
Gene difference in methylation cycle function can be a factor in risk for sulfur metabolism dysfunction. Excess dietary intake can be causal too. Sulfites are used as food preservatives and also naturally increases in dried foods like dried mushrooms or fruit. Hydrogen sulfide is protective against TNF-alpha though. (Diaz Sanchez, et al., 2023) Sulfur metabolism helps us with detoxification of many chemicals, but sulfur metabolism can cause toxic effects if sulfur chemicals aren’t broken down.

Lachnospiraceae are a standard gut microbial species, and member of the group Firmicutes. They do produce short chain fatty acids for us, but the species can also be associated with disease conditions. Having a ratio of more Bacteroidetes to Firmicutes species is associated with less risk for obesity. Pomegranate peel promotes more of the Bacteroidetes in ratio to Firmicutes. Pomegranate promotes balanced growth of species that produce butyrate and short chain fatty acids (SCFA), improving the ratio of Bacteroidetes to Firmicutes. Rosaburia and Blauta were increased and also are SCFA producing species. (George, et al, 2019)
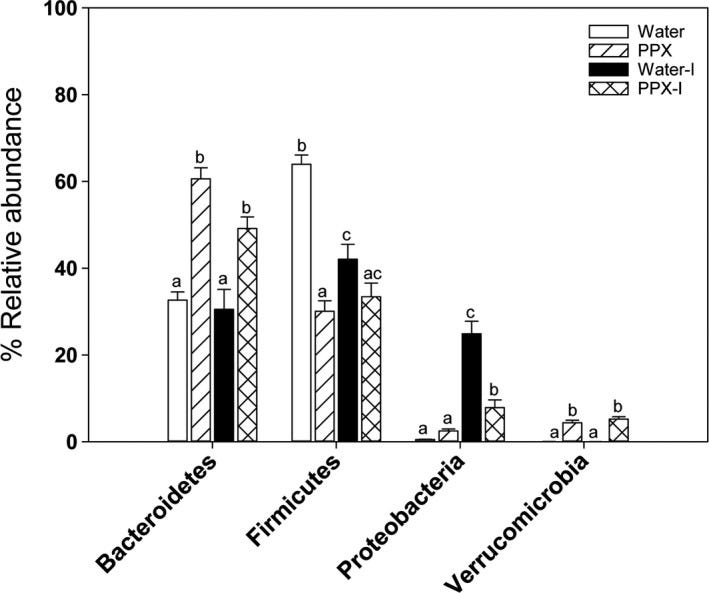
“PPX treatment decreased the Firmicutes/Bacteroidetes ratio by increasing Bacteroidetes and decreasing Firmicutes levels. The decrease in Firmicutes was driven by a large reduction in Lactobacillus. PPX treatment increased the abundance of Proteobacteria and Verrucomicrobiae and decreased Actinobacteria.” […] “Fruit extracts, rich in polyphenols, including pomegranate, stimulated the growth of Akkermansia municiphila (Anhe, Roy, Pilon, & Dudonne, 2015; Henning, Summanen, Lee, & Yang, 2017; Li, Henning, et al., 2015; Zhang et al., 2018) which is thought to have beneficial health effects (Ottman, Geerlings, Aalvink, Vos, & Belzer, 2017). An increase in Escherichia coli (E. coli), enterobacteria, and total aerobic counts was found in feces from DSS‐treated rats that decreased in response to treatment with pomegranate extract, or its metabolite urolithin A (Larrosa et al., 2010). Analysis of data from 16S rRNA gene sequencing showed a significant increase in the family Ruminococcaceae in feces from DSS‐treated rats receiving pomegranate extract compared with DSS‐treated rats receiving water (Kim, Banerjee, Sirven, & Minamoto, 2017).” (George, et al, 2019)
Pomegranate peel extract treatment helped protect against exposure to an infectious pathogen and shifted the gut microbiome balance of Bacteroidetes to Firmicutes species towards Bacteroidetes. (George, et al, 2019)
“GI microbes produce several bioactive compounds which can influence the physiology of the host [6,7]; some, like vitamins, are beneficial [8], whilst others are toxic [9]. The Human Microbiome Project and MetaHit have led to an improved overview of the human-associated microbial repertoire [10,11]. The compiled data from these studies revealed that the human microbiota comprises 12 different phyla, of which 93.5% belong to Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria. Among these, Firmicutes and Bacteroidetes dominate the gut microbiota in healthy subjects [12]. The Lachnospiraceae family is a phylogenetically and morphologically heterogeneous taxon belonging to the clostridial cluster XIVa of the phylum Firmicutes (Figure 1) [13].” (Vacca, et al., 2020)
Bacteroidetes and Melatonin (Brave AI summary)
Melatonin has been shown to influence the levels of Bacteroidetes in the gut microbiota. Studies indicate that melatonin can decrease the ratio of Firmicutes to Bacteroidetes, which is often associated with improved intestinal health. For example, in a study involving mice subjected to sleep deprivation and stress, melatonin was found to decrease the Firmicutes to Bacteroidetes ratio and reduce the abundance of Proteobacteria at the phylum level. Additionally, melatonin increases the abundance of beneficial bacteria such as Akkermansia, Lactobacillus, and Faecalibacterium, while decreasing harmful bacteria like Aeromonas. (Iesanu, et al., 2022)
“Major interactions have been identified between the gut microbiota and melatonin, where enteric communities contribute to the biotransformation and metabolism of tryptophan to serotonin and eventually to melatonin [19], while this indolamine has been shown to have a beneficial effect on intestinal barrier function and microbial communities [20,21].” (Iesanu, et al., 2022)
In another study, melatonin supplementation in mice fed a high-fat diet led to an increase in the abundance of Bacteroidetes and a decrease in Firmicutes. This suggests that melatonin may help prevent obesity by modulating the gut microbiota, specifically by decreasing the Firmicutes to Bacteroidetes ratio and increasing the abundance of Akkermansia. (Brave AI summary; Iesanu, et al., 2022; Park, et al., 2020)
“Compared to the control, melatonin concentration was lower in the WS and SD. Fecal concentration was 0.132 pg/mL in control, 0.062 pg/mL in WS, and 0.068 pg/mL in SD. In colon tissue, it was 0.45 pg/mL in control, 0.007 pg/mL in WS, and 0.03 pg/mL in SD. After melatonin treatment, melatonin concentrations in feces and colon tissue were recovered to the level of control. Metagenomic analysis of microbiota showed abundance in colitogenic microbiota in WS and SD. Melatonin injection attenuated this harmful effect. WS and SD showed decreased Lactobacillales and increased Erysipelotrichales and Enterobacteriales. Melatonin treatment increased Akkermansia muciniphila and Lactobacillus and decreased Bacteroides massiliensis and Erysipelotrichaceae.” (Park, et al., 2020)
Note that Akkermansia muciniphila is a species associated with living over 100 years old. It helps our membrane’s biofilm layer to remain protective instead of getting too thick or too thin.
Gut Bacteria and Sleep Association (Brave AI summary)
Several gut bacterial species have been associated with better sleep quality. Here are some of the key species:
Bifidobacterium: A study published in the journal Sleep found that children with a high concentration of bifidobacterium bacteria had more time asleep during the night, indicating a positive relationship between this bacteria and sleep quality.
Bacteroides: This bacterial species was found to be higher in children who had a greater amount of time asleep compared to the time in bed trying to sleep, suggesting it may also contribute to better sleep patterns. (Bacteroidetes; Smith, et al., 2019)
Lactobacillus: While not specifically mentioned in the context of sleep in the provided sources, studies have suggested that certain strains of Lactobacillus may help enhance sleep quality, particularly in terms of sleep induction and reducing subclinical signs of anxiety and depression.
Akkermansia: Supplementation with melatonin to sleep-deprived mice has been shown to increase the abundance of Akkermansia, indicating a potential positive role in sleep quality.
These findings suggest that a diverse and balanced gut microbiome, rich in beneficial bacteria like Bifidobacterium, Bacteroides, Lactobacillus, and Akkermansia, may contribute to better sleep quality.
Pomegranate peel would help promote Bacteroidetes in balance with Firmicutes and therefore may be helping us with abstract thinking!
“We found that total microbiome diversity was positively correlated with increased sleep efficiency and total sleep time, and was negatively correlated with wake after sleep onset. We found positive correlations between total microbiome diversity and interleukin-6, a cytokine previously noted for its effects on sleep. Analysis of microbiome composition revealed that within phyla richness of Bacteroidetes and Firmicutes were positively correlated with sleep efficiency, interleukin-6 concentrations and abstract thinking. Finally, we found that several taxa (Lachnospiraceae, Corynebacterium, and Blautia) were negatively correlated with sleep measures.” (Smith, et al., 2019)
New Study Shows What's in Your Gut Influences How and When You Sleep | Sleep Foundation
Changes in sleep timing of as little as 90 minutes difference in the average midpoint can negatively affect the gut microbiome species towards dysbiosis.
Look into ankyrin repeat domains, chimeric spike S1 can bind it or mess with it. https://alz-journals.onlinelibrary.wiley.com/doi/abs/10.1002/alz.047572…
Sunshine in the day counts - for mitochondria, gut microbiome, and better sleep:
“HEALTH ALERT: WHY SHOULD YOU WORK CLOSE TO A WINDOW? Have you read the "with or without windows" study? This study compared workers who had a workspace with windows to workers who had a workspace without windows. And what it found was.....
Workers who had a window near them during the day had lower cortisol and higher melatonin levels at night (when they are supposed to) compared to the workers who did not have windows during the day. So the workers who did not have a window at their work had lower melatonin and higher cortisol levels at night.
This is bad for your physical and mental health because higher cortisol levels are positively correlated with minor psychiatric disorders and depressive symptoms at 10:00 pm. Lower melatonin levels at 10:00 pm were correlated with depressive symptoms and poor quality of sleep.
Work outside if you can but if you can't, at least try to be near a window. And if that is truly impossible then you have to hack it with the red, infrared, UV and bright light therapy machines. I'll link to my favs below.
"Our study demonstrated that not only may light pollution affect human physiology but also lack of exposure to natural light is related to high levels of cortisol and lower levels of melatonin at night, and these, in turn, are related to depressive symptoms and poor quality of sleep.”” (Harb, et al., 2014) (x.com/BlueLightDiet)
Disclaimer: This information is being shared for educational purposes within the guidelines of Fair Use and is not intended to provide individual health care guidance.
Reference List
(Chen, et al., 2020a) Chen, D., Mei, Y., Lan, G., Gan, C.-L., Zhang, T., Xia, Y., Wang, L. and Lee, T.H. (2020), Novel regulation of death-associated protein kinase 1 in Alzheimer’s disease. Alzheimer's Dement., 16: e047572. https://doi.org/10.1002/alz.047572 https://alz-journals.onlinelibrary.wiley.com/doi/abs/10.1002/alz.047572
(Chen, et al., 2020b) Chen D, Mei Y, Kim N, Lan G, Gan CL, Fan F, Zhang T, Xia Y, Wang L, Lin C, Ke F, Zhou XZ, Lu KP, Lee TH. Melatonin directly binds and inhibits death-associated protein kinase 1 function in Alzheimer's disease. J Pineal Res. 2020 Sep;69(2):e12665. doi: 10.1111/jpi.12665. Epub 2020 May 27. PMID: 32358852; PMCID: PMC7890046. https://pmc.ncbi.nlm.nih.gov/articles/PMC7890046/
(Clark and Vissel, 2014) Clark, I.A., Vissel, B. Inflammation-sleep interface in brain disease: TNF, insulin, orexin. J Neuroinflammation 11, 51 (2014). https://doi.org/10.1186/1742-2094-11-51 https://jneuroinflammation.biomedcentral.com/articles/10.1186/1742-2094-11-51
(Diaz Sanchez, et al., 2023) Diaz Sanchez L, Sanchez-Aranguren L, Wang K, Spickett CM, Griffiths HR, Dias IHK. TNF-α-Mediated Endothelial Cell Apoptosis Is Rescued by Hydrogen Sulfide. Antioxidants. 2023; 12(3):734. https://doi.org/10.3390/antiox12030734 https://www.mdpi.com/2076-3921/12/3/734
(Harb, et al., 2014) Harb, F., Hidalgo, M. P., & Martau, B. (2014). Lack of exposure to natural light in the workspace is associated with physiological, sleep and depressive symptoms. Chronobiology International, 32(3), 368–375. https://doi.org/10.3109/07420528.2014.982757 https://www.tandfonline.com/doi/10.3109/07420528.2014.982757
(Huang, et al., 2019) Huang CC, Chiou CH, Liu SC, Hu SL, Su CM, Tsai CH, Tang CH. Melatonin attenuates TNF-α and IL-1β expression in synovial fibroblasts and diminishes cartilage degradation: Implications for the treatment of rheumatoid arthritis. J Pineal Res. 2019 Apr;66(3):e12560. doi: 10.1111/jpi.12560. Epub 2019 Feb 14. PMID: 30648758. https://pubmed.ncbi.nlm.nih.gov/30648758/
(Iesanu, et al., 2022) Iesanu MI, Zahiu CDM, Dogaru IA, Chitimus DM, Pircalabioru GG, Voiculescu SE, Isac S, Galos F, Pavel B, O'Mahony SM, Zagrean AM. Melatonin-Microbiome Two-Sided Interaction in Dysbiosis-Associated Conditions. Antioxidants (Basel). 2022 Nov 14;11(11):2244. doi: 10.3390/antiox11112244. PMID: 36421432; PMCID: PMC9686962. https://pmc.ncbi.nlm.nih.gov/articles/PMC9686962/
(Li, et al., 2006) Li, J., Mahajan, A., Tsai, M.-D., Ankyrin Repeat: A Unique Motif Mediating Protein−Protein Interactions, J of Biochemistry, 2006/12/01, Vol 45(51), pp 15168-15178, https://doi.org/10.1021/bi062188q https://pubs.acs.org/doi/10.1021/bi062188q
(Park, et al., 2020) Park YS, Kim SH, Park JW, Kho Y, Seok PR, Shin JH, Choi YJ, Jun JH, Jung HC, Kim EK. Melatonin in the colon modulates intestinal microbiota in response to stress and sleep deprivation. Intest Res. 2020 Jul;18(3):325-336. doi: 10.5217/ir.2019.00093. Epub 2020 Jun 23. PMID: 32564539; PMCID: PMC7385569. https://pmc.ncbi.nlm.nih.gov/articles/PMC7385569/
(Patterson, et al., 2024) Patterson, E., Tan, H.T.T., Groeger, D. et al. Bifidobacterium longum 1714 improves sleep quality and aspects of well-being in healthy adults: a randomized, double-blind, placebo-controlled clinical trial. Sci Rep 14, 3725 (2024). https://doi.org/10.1038/s41598-024-53810-w https://www.nature.com/articles/s41598-024-53810-w
(sciencedaily.com), King’s College London, New research has found irregular sleep patterns are associated with harmful bacteria in your gut., August 2, 2023, https://www.sciencedaily.com/releases/2023/08/230802003415.htm
(Smith, et al., 2019) Smith RP, Easson C, Lyle SM, Kapoor R, Donnelly CP, Davidson EJ, Parikh E, Lopez JV, Tartar JL. Gut microbiome diversity is associated with sleep physiology in humans. PLoS One. 2019 Oct 7;14(10):e0222394. doi: 10.1371/journal.pone.0222394. PMID: 31589627; PMCID: PMC6779243. https://pmc.ncbi.nlm.nih.gov/articles/PMC6779243/
(Tavartkiladze, et al., 2024) : Tavartkiladze, A., Simonia, G., Lou, R., Revazishvili, P., Maisuradze, M., et al. (2024). Low Melatonin and Post-COVID Syndrome: Unveiling SIBO as a Potential Pre-Cancerous Condition in the Post-Pandemic Era. J Cancer Res, 2(1), 1-12. https://www.wecmelive.com/open-access/low-melatonin-and-post-covid-syndrome-unveiling-sibo-as-a-potential-pre-cancerous-condition-in-the-postpandemic-era.pdf
(Tenore, et al., 2018) Tenore, Gian Carlo & Bottone, S. & Riccio, Gennaro & Badolati, Nadia & Stornaiuolo, Mariano & Novellino, Ettore. (2018). A Crosstalk between Melatonin and Taste-Receptors’ Signaling Tunes Quinine-Induced Gut Hormone Secretion in Mice. Journal of Nutrition & Food Sciences. 08. 10.4172/2155-9600.1000664. https://www.researchgate.net/publication/323137169_A_Crosstalk_between_Melatonin_and_Taste-Receptors'_Signaling_Tunes_Quinine-Induced_Gut_Hormone_Secretion_in_Mice
(Vacca, et al., 2020) Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms. 2020 Apr 15;8(4):573. doi: 10.3390/microorganisms8040573. PMID: 32326636; PMCID: PMC7232163. https://pmc.ncbi.nlm.nih.gov/articles/PMC7232163/
(Wu, et al., 2021) Wu S, Lu W, Cheng G, et al. DAPK1 may be a potential biomarker for arterial aneurysm in clinical treatment and activated inflammation levels in arterial aneurysm through NLRP3 inflammasome by Beclin1. Human & Experimental Toxicology. 2021;40(12_suppl):S563-S572. doi:10.1177/09603271211041667 https://journals.sagepub.com/doi/10.1177/09603271211041667
Functionally, the ankyrin repeat domain is found in many different proteins, but it does many different things for those proteins rather than having a consistent function. (Li, et al., 2006)








Thank you for this very informative research article. Could you possibly provide more information on thyroid issues and bone health? And again, sinerely and appreciatively thank you : )