Liver injury & comorbid fibromyalgia, by way of Retinoid Toxicity; also autoimmune thyroiditis
Hashimoto's thyroiditis is an autoimmune condition that may have hypo or hyperthyroid levels of thyroid hormone production. It is also frequently comorbid with fibromyalgia.
Fibromyalgia is a chronic fatigue and pain condition which is not well understood and was dismissed, or is dismissed, by healthcare providers as being not real, or nowhere near as severe a problem as more visible problems. Mitochondrial dysfunction seems involved in the fatigue. The pain can be widespread, diffuse aches, or more specific muscle knots, cramps that remain firm and can limit the range of motion. Excess phosphorus compared to calcium and magnesium has been theorized to be involved but lab tests tend to not show specific ‘provable’ problems.
Average appointment for someone with chronic symptoms of fibromyalgia (imagine years of mystery symptoms): “Your lab tests were normal, (you must be a hypochondriac to be complaining so much); why don’t you go see a talk therapist and discuss stress coping skills?”
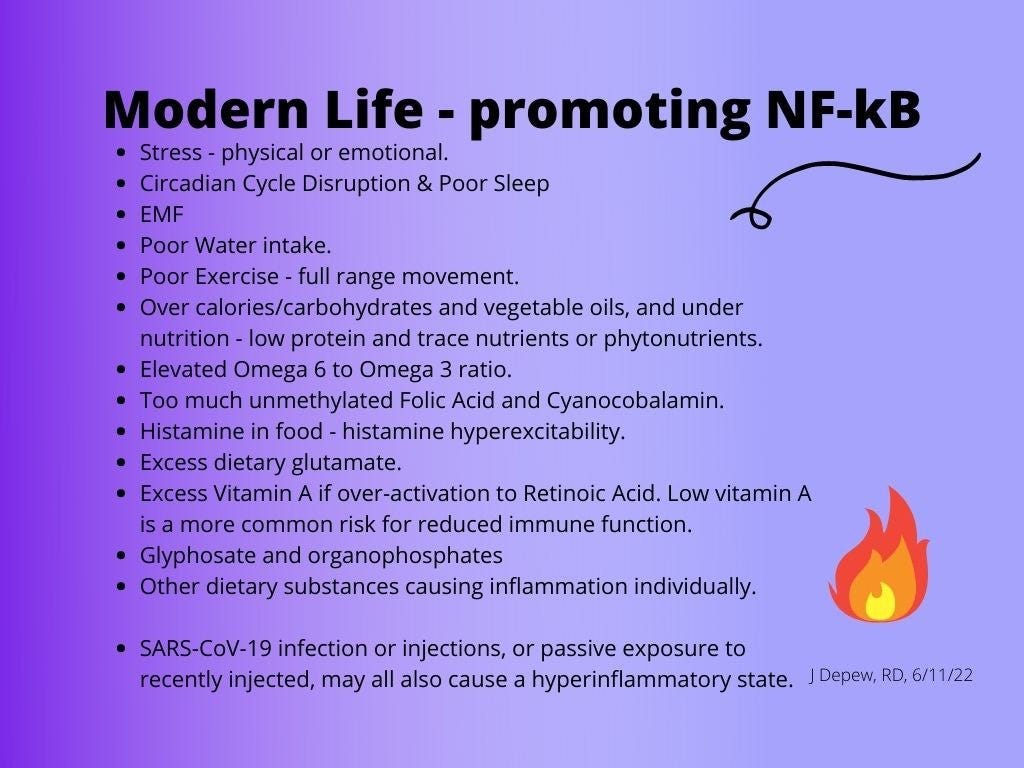
The stress coping skills would be helpful as physical or emotional stress adds to the chronic inflammation which is part of the symptom severity. Any reduction in overall stress load would help reduce symptoms somewhat. But »> tackling the root causal issue, however, would still be needed for the body to be able to return to normal function. Skin doesn’t grow normally when there is an excess of activated retinoids - the gene transcription, hormone-like form of vitamin A. It is more inflamed like tissue trying to fight an infection of heal a wound - but there doesn’t need to be an infection or wound if the body as too much active Retinoic Acid.
An excess of active retinoids may occur from medication use - and is what has been studied. The majority of research available about Retinoid Toxicity is from medication side effects which might be cosmetic or cancer medications.
Retinoid toxicity can result from damage to the liver from injury, or disease - cirrhosis or fatty liver disease, or infection - Hepatitis C, and fibromyalgia has been associated with liver conditions such as cirrhosis. (Rogal, et al., 2015) Epstein-Barr Virus, (also known as mononucleosis), has been found to cause chronic Retinoid Toxicity due to a changed gene for a liver enzyme which activates vitamin A.
Reducing additional sources of vitamin A and carotenoids from the diet can help reduce symptoms of an excess of bioactive retinoids. If the body doesn’t have a flu to fight, why dress up in the congested nose, itchy eyes, and achy muscles?
Are there specific liver enzymes or biomarkers that can aid in diagnosing fibromyalgia in patients with liver disease? - The Brave AI summary lists several known for liver disease or cirrhosis, one of which may be involved in Retinoid Toxicity:
α-smooth muscle actin: This marker correlates with activation of myofibroblasts and is a reliable indicator of hepatic stellate cells (HSCs) activation, which may be relevant to liver fibrosis. However, its connection to fibromyalgia is indirect. **HSCs may be involved in Retinoid Toxicity.
Key Takeaways regarding Fibromyalgia comorbidity with liver injury
Higher prevalence of fibromyalgia among patients with chronic hepatitis C virus (HCV) infection (35%) and NASH (30%), than for alcohol-related liver disease (12%), but all were more likely to have comorbid fibromyalgia than the average population (5%, [4]). (Rogal, et al., 2015)
Association with increased pain intensity, tender point count, symptom severity, and fatigue
Not related to viral load of HCV.
Systemic inflammation and pro-inflammatory cytokine profiles may play a role
Addressing liver disease and psychiatric comorbidities is essential for managing pain and symptoms
The following excerpt is a description of the nerve signaling involved in fibromyalgia pain, which is widespread, suggesting a central mechanism rather than isolated muscle knots. The paragraph concludes with the suggestion that chronic liver disease also has pain and that might lead to the worse fibromyalgia pain becoming comorbid with a liver condition. But, or . . . or the liver disease might be leading to increased active retinoids in widespread circulation leading to inflammatory pain signaling everywhere …
“The current data support a critical role for central sensitization in the development of fibromyalgia. In this model, the processing of peripheral pain is amplified by the central nervous system via excitatory amino acids, substance P, and neurotrophins, and abnormalities of the autonomic nervous system and neuroendocrine axis along with negative affect participate in this amplification [16]. A recent study demonstrated that localized pain was among the strongest predictors of subsequent widespread pain, indicating a possible central mechanism [17]. We have previously found that pain, particularly abdominal pain, is common in patients with chronic liver disease and may increase as liver disease advances [18]. The pain that is common in cirrhosis may predispose to fibromyalgia symptoms.”
Medical professional: “On the body-shaped graphic, point to where you feel pain.”
Me: “All of it. Everywhere. Even my hair follicles hurt.”
[*During a flair-up, not currently. Previous post]
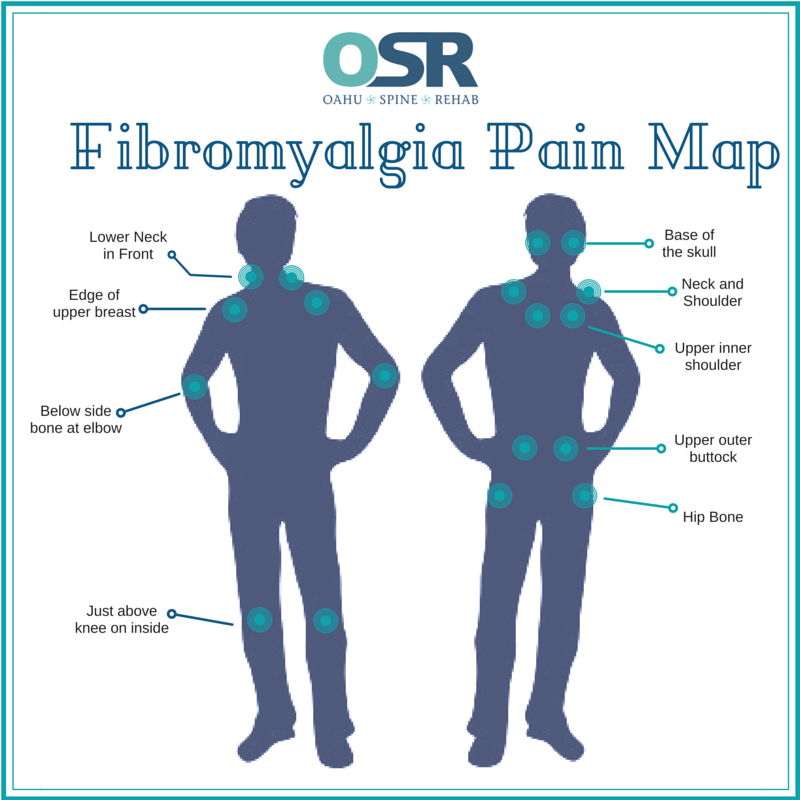
Who should care about a link between fibromyalgia, (Hashimoto’s thyroiditis) and liver disease? or why should we care?
An epidemic rate increase in non-alcoholic fatty liver disease is occurring, and this information suggests that fibromyalgia pain and fatigue might be waiting just a little way down the road.
Non-alcoholic fatty liver disease (NAFLD) has become much more common and starting at young ages, even in teenagers. The industry switch to use of high fructose sweetener for soda and other beverages is likely a big factor. Progression of NAFLD may be rapid compared to previous older onset cases. It is a part of the same group of chronic liver disease as non-alcoholic steatohepatitis (NASH).
Vitamin A metabolism can occur in many types of cells within the body, however a majority takes place within the liver in specialized cells called hepatic stellate cells (HSCs). The balance or ration of different forms of retinoids affects the function of the hepatic stellate cells. Traumatic damage to the liver can cause a physical dumping of retinoids into the blood stream and other forms of liver disease can also cause an increase in activated retinoids being released into the body. More detail is included in a later section of this post.
“Retinoids, especially the ATRA metabolite, are very potent transcriptional regulators that help control expression of more than 500 diverse genes.2 A role for retinoids in preventing or blocking hepatic disease development has long been recognized.” (Blaner, 2019)
Active vitamin A is hormone like and affects our production of many genes that are involved in our immune responses and wound healing capabilities. Retinoid toxicity tends to include chapped lips and eczema because skin growth is abnormal - it is trying to heal a wound that isn’t there and never finishes the job if the daily diet is adding more vitamin A and carotenoids into a system that is releasing too much of it into the bloodstream in activated forms.
A damaged liver might be releasing an excess of retinoids, but an infection can also be a cause of over-activation by a liver enzyme. Epstein-Barr virus can cause a gene change that causes that to happen continually - even once the infection is contained, it seems to me. Chronic fatigue Syndrome has been associated with history of an Epstein Barr viral infection. SARS-CoV-2 chimeric spike also seems to have that capability.
Retinoid Toxicity due to liver damage may also be a causal factor in symptoms resembling “COVID19”.
“This article outlines a new hypothesis of the nature of the inflammatory factor responsible for tissue damage in COVID-19. Review of the literature reveals that COVID-19 disease closely resembles an endogenous form of hypervitaminosis A. We propose that SARS-CoV-2 virus-induced liver damage causes retinoic acid and stored retinyl esters to be released into the circulation in toxic concentrations, unbound to protein, with resulting damage to organs including the lungs, heart, blood vessels, and skin.” (Mawson, et al., 2021)
Another clue: Fibromyalgia has also been associated with thyroid conditions.
Fibromyalgia associated with Hashimoto thyroiditis - 30-40% estimated, 62% in this study by (Haliloglu, et al., 2017), discussion of it follows this introduction.
Tying some loose ends together first, more detail is included in later sections, what is the bigger picture of these comorbid conditions? How are they linked? Coincidence is not statistically probable.
Not only is fibromyalgia more likely to occur with liver injuries,
and fibromyalgia is more likely to occur with Hashimoto’s thyroiditis than in the average population,
but also, having Hashimoto thyroiditis is more likely to lead to liver injury.
That gives us a circle . . .
. . . and suggests to me that the causal links (since coincidence is improbable) might be
that a lack of selenium and endogenous glutathione production and polyphenols leads to »>
autoimmune thyroid conditions, which can lead to »>
liver injury, which might be an underlying cause of »>
fibromyalgia from an excess of activated retinoids being released from the damaged liver into the bloodstream.
And chronic retinoid excess can lead to liver, kidney and brain damage (Alzheimer’s dementia from hippocampal damage). Cancer and other risks caused by methylation dysfunction like Parkinson’s Disease might also occur.
See the Fibromyalgia Spiral graphic below.

The way to stop the escalating spiral of chronic fatigue, pain, and organ damage, is to increase anti-inflammatory function and decrease inflammatory signaling.
Increase anti-inflammatory antioxidant and mitochondrial nutrient support:
add more selenium and antioxidants to the diet, as supplements, and mitochondrial & methylation support nutrients and enough protein to provide amino acids for endogenous glutathione production;
increase intake of Nrf2 promoters that aren’t rich carotenoid sources;
increase niacin and zinc, resistant starch and pomegranate peel and promote butyrate species in the gut microbiome;
improve sleep and darkness/sunshine in support of circadian cycle function and night-time detox of the brain (glymphatic system);
drink water or herbal tea and other non-caffeinated beverages;
get exercise and full range of motion stretching.
The above factors are also helping promote BDNF and reduce deuterium concentration.
Decrease inflammation and reduce vitamin A and carotenoid availability:
depending on severity of the symptoms, it may be necessary to strictly limit vitamin A and carotenoids, glutamate and histamine sources;
decrease any emotional or physical stressors, or as many as possible - this includes EMF exposure and smog/bad air, and flickering lights/action movies too if histamine excess is a problem.
Adding extra antioxidants won’t be enough of a solution once the inflammatory signaling is already happening. The escalating spiral needs to be blocked.

For more about the above two graphics, see this post, which includes more detail about the lifestyle and diet changes that may help:
Fibromyalgia & Hashimoto thyroiditis:
Abstract: Fibromyalgia (FM) is a syndrome characterised by chronic musculoskeletal pain, tenderness and other somatic symptoms. The prevalence of FM is approximately 2–7% in the general global population and is 30–40% in the population of Hashimoto thyroiditis (HT) with a structural pathology. In 2010, new classification criteria for FM were proposed, as an alternative to the American College of Rheumatology (ACR) 1990 criteria. The objectives of the present study were to identify the prevalence of FM in the HT population and evaluate the associated features by using the new diagnostic criteria. The study group included 79 consecutive patients with HT with or without FM. Recorded data included age, gender, laboratory parameters, sociodemographic features and clinical findings, presence of somatic symptoms, and disease activity indices. The prevalence of FM in patients with HT was 62%. Antithyroid peroxidase antibody (TPOAb) positivity, duration of disease, and waist circumference were significantly associated with concomitant FM (p = 0.000, p = 0.000, and p = 0.015, respectively).
A strong positive correlation was noted between fibromyalgia impact questionnaire (FIQ) scores and disease duration, age, values of thyroid-stimulating hormone (TSH) and TPOAb, waist circumference and marital status. TPOAb was found to be independent of body mass index, age and TSH. (Haliloglu, et al., 2017)
Revisualizing the academic wording »> The people in the study who had both fibromyalgia and Hashimoto’s thyroiditis were more likely to have been symptomatic for a greater number of years; were likely to be older and married; they had higher TSH and TPOAb values, which suggests worse autoimmune disease (the TPOAb) and maybe lower iodine levels (the TSH); and they had a larger waist circumference which might mean more overweight but it might also mean more bloated with gas and water from Retinoids over activating TRP channels leading to puffy edema and poor digestion.
The TPOAb autoimmune severity was independent of - not correlated with higher or lower BMI, older or younger age, or with the level of TSH, thyroid stimulating hormone.
From the earlier series of posts about Hashimoto’s thyroiditis (HT) readers of this Substack learned that a lack of glutathione and selenium is involved with autoimmune thyroiditis. Lack of antioxidant protection within the thyroid gland causes mitochondrial harm from oxidative stress promoted during iodine uptake. Iodine deficiency might be present in HT, but isn’t always a factor. Lack of antioxidants like selenium and endogenous glutathione was more consistent with autoimmune HT than iodine deficiency. Polyphenols are also protective.
An elevated TSH though can be seen with iodine deficiency. (Brave AI summary)
“Iodine deficiency results in inadequate production of T4. In response to decreased blood T4 concentrations, the pituitary gland increases its output of TSH. Persistently elevated TSH levels may lead to hypertrophy (enlargement) of the thyroid gland, also known as goiter (see Deficiency) (3).” (lpi.oregonstate.edu)
The conclusion of the Abstract has the sensible point that treatment success for HT patients could improve if we consider the patient’s fibromyalgia status too - *and it may be affecting 62% of autoimmune thyroid patients.
Concomitant FM is a common clinical problem in HT and its recognition is important for the optimal management of the disease. The new set of diagnostic criteria for FM reinforces this situation. Consideration of the FM component in the management of HT increases the likelihood of treatment success. (Haliloglu, et al., 2017)
Fibromyalgia comorbid with liver disease - Brave AI
Fibromyalgia (FM) is a chronic condition characterized by widespread musculoskeletal pain, fatigue, and tender points. Liver disease, including chronic hepatitis C virus (HCV) infection, non-alcoholic steatohepatitis (NASH), and alcohol-related liver disease, has been found to be comorbid with FM.
Prevalence and Characteristics
Studies suggest a higher prevalence of fibromyalgia among patients with chronic Hepatitis C Virus infection or NASH than for alcohol-related liver disease. One study found that 35% of patients with HCV had FM, compared to 12% of those with alcohol-related liver disease and 30% of those with non-alcoholic steatohepatitis (NASH). (Rogal, et al., 2015)
“Fibromyalgia symptoms were significantly associated with etiology of liver disease (HCV: 35 %, NASH: 30 %, alcohol-related liver disease: 12 %, p < 0.01).”
“Fibromyalgia symptoms were significantly associated with HCV and NASH cirrhosis and with psychiatric symptoms. Future work should focus on the underlying pathophysiology and management of widespread pain in patients with cirrhosis.” (Rogal, et al., 2015)
FM in patients with liver disease is associated with increased pain intensity, tender point count, symptom severity, and fatigue. Notably, the prevalence of FM does not appear to be related to the viral load of HCV but more to do with the liver disease, sleep issues and psychiatric symptoms were mentioned. (Rogal, et al., 2015)
“Sleep disorders, psychiatric disease, and elevated levels of inflammatory cytokines are associated with both fibromyalgia and cirrhosis. Disordered sleep is a hallmark of both hepatic encephalopathy and fibromyalgia, with sleep disturbances thought to predispose people to more severe pain [6, 7]. Mood disorders that have been linked to fibromyalgia [8] are common in cirrhosis [9]. The role of inflammation remains unclear in fibromyalgia, but pro-inflammatory cytokines have been elevated in some studies [10]. Similarly, nonalcoholic steatohepatitis (NASH) [11], alcohol-related liver disease [12–14], and HCV [15] have all been associated with elevations in pro-inflammatory cytokines compared with controls.” (Rogal, et al., 2015)
»> We detox while we sleep - and we can’t do that as well if we aren’t sleeping well.
The glymphatic system of the brain is much more active at night. «<
Pathogenic Role
The exact mechanisms underlying the comorbidity between FM and liver disease are unclear. However, it is thought that systemic inflammation and pro-inflammatory cytokine profiles may play a role. Addressing liver disease and psychiatric comorbidities, such as depression and anxiety, is essential for managing pain and symptoms.
Clinical Implications
Healthcare providers should be aware of the increased risk of FM among patients with liver disease, particularly those with HCV infection and NASH. Early recognition and treatment of FM can improve quality of life and reduce symptom burden for these patients.
sciencedirect.com, Fibromyalgia syndrome in chronic hepatitis C virus (HCV) infection patients: A potential association and pathogenic role - ScienceDirect
researchgate.net, Fibromyalgia Symptoms and Cirrhosis | Request PDF
Retinoid toxicity and hepatic stellate cells - Brave AI
Retinoid toxicity can affect hepatic stellate cells (HSCs), which are responsible for storing and releasing retinoids (vitamin A) in the liver. HSCs play a crucial role in regulating retinoid levels, and alterations in their function or morphology can impact retinoid homeostasis.
Abstract: “Vitamin A (retinol) is important for normal growth, vision and reproduction. It has a role in the immune response and the development of metabolic syndrome. Most of the retinol present in the body is stored as retinyl esters within lipid droplets in hepatic stellate cells (HSCs). In case of liver damage, HSCs release large amounts of stored retinol, which is partially converted to retinoic acid (RA). This surge of RA can mediate the immune response and enhance the regeneration of the liver. If the damage persists activated HSCs change into myofibroblast-like cells producing extracellular matrix, which increases the chance of tumorigenesis to occur. RA has been shown to decrease proliferation and metastasis of hepatocellular carcinoma. The levels of RA and RA signaling are influenced by the possibility to esterify retinol towards retinyl esters. This suggests a complex regulation between different retinoids, with an important regulatory role for HSCs.” (Haaker, et al., 2020)
Changes in Retinoid Content
HSCs’ retinoid content changes depending on nutrition and stress levels. In response to liver injury, HSCs undergo transdifferentiation into myofibroblastic cells, leading to loss of lipid droplets and retinoid storage. This process can affect retinoid levels and potentially contribute to retinoid toxicity.
Regulation of Retinoid Levels
HSCs regulate retinoid levels through esterification of retinol to retinyl esters, which are stored in lipid droplets. This complex regulation involves interactions between different retinoids and HSCs. Disruptions in this regulation can lead to retinoid toxicity.
Consequences of Retinoid Toxicity
Retinoid toxicity can have severe consequences, including:
Impaired retinoid absorption and storage
Changes in HSC morphology and function
Increased risk of liver fibrosis and carcinogenesis
Key Findings
Hepatic stellate cells retain retinoid-laden lipid droplets after transdifferentiation into activated myofibroblasts (Jophlin et al., 2018)
Absence of HSC retinoid lipid droplets decreases hepatic carcinogenesis but not fibrosis (Kluwe et al., 2015)
Dietary retinoid status strongly regulates stellate cell lipid droplet composition, while triglyceride intake has no significant effect (Erdogan et al., 1987)
In summary, retinoid toxicity can impact hepatic stellate cells, leading to changes in retinoid storage and regulation, and potentially contributing to liver disease. Understanding the complex interactions between retinoids and HSCs is essential for elucidating the mechanisms of retinoid toxicity and developing effective therapeutic strategies.
Follow up
nature.com, Single cell analysis in native tissue: Quantification of the retinoid content of hepatic stellate cells | Scientific Reports
Blaner, William S. Ph.D.*,1. Hepatic Stellate Cells and Retinoids: Toward A Much More Defined Relationship. Hepatology 69(2):p 484-486, February 2019. | DOI: 10.1002/hep.30293 journals.lww.com, Hepatic Stellate Cells and Retinoids: Toward A Much More... : Hepatology “Retinoids, especially the ATRA metabolite, are very potent transcriptional regulators that help control expression of more than 500 diverse genes.2 A role for retinoids in preventing or blocking hepatic disease development has long been recognized.” (Blaner, 2019)
Bobowski-Gerard M, Zummo FP, Staels B, Lefebvre P, Eeckhoute J. Retinoids Issued from Hepatic Stellate Cell Lipid Droplet Loss as Potential Signaling Molecules Orchestrating a Multicellular Liver Injury Response. Cells. 2018 Sep 13;7(9):137. doi: 10.3390/cells7090137. PMID: 30217095; PMCID: PMC6162435. ncbi.nlm.nih.gov, Retinoids Issued from
Moriwaki H, Blaner WS, Piantedosi R, Goodman DS. Effects of dietary retinoid and triglyceride on the lipid composition of rat liver stellate cells and stellate cell lipid droplets. J Lipid Res. 1988 Nov;29(11):1523-34. PMID: 3241127. pubmed.ncbi.nlm.nih.gov, Effects of dietary retinoid a
Disclaimer: This information is being provided for educational purposes within the guidelines of Fair Use and is not intended to provide individual health care guidance.
Reference List
(Haaker, et al., 2020) Haaker MW, Vaandrager AB, Helms JB. Retinoids in health and disease: A role for hepatic stellate cells in affecting retinoid levels. Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Jun;1865(6):158674. doi: 10.1016/j.bbalip.2020.158674. Epub 2020 Feb 24. PMID: 32105672. https://www.sciencedirect.com/science/article/pii/S1388198120300664?via%3Dihub
(Haliloglu, et al., 2017) Haliloglu S, Ekinci B, Uzkeser H, Sevimli H, Carlioglu A, Macit PM. Fibromyalgia in patients with thyroid autoimmunity: prevalence and relationship with disease activity. Clin Rheumatol. 2017 Jul;36(7):1617-1621. doi: 10.1007/s10067-017-3556-2. Epub 2017 Feb 7. PMID: 28176037. https://link.springer.com/article/10.1007/s10067-017-3556-2
(Mawson, et al., 2021) Mawson AR, Croft AM, Gonzalez-Fernandez F. Liver Damage and Exposure to Toxic Concentrations of Endogenous Retinoids in the Pathogenesis of COVID-19 Disease: Hypothesis. Viral Immunol. 2021 Jul-Aug;34(6):376-379. doi: 10.1089/vim.2020.0330. Epub 2021 May 13. PMID: 33983857; PMCID: PMC8392079. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8392079/
(Rogal, et al., 2015) Rogal SS, Bielefeldt K, Wasan AD, Szigethy E, Lotrich F, DiMartini AF. Fibromyalgia symptoms and cirrhosis. Dig Dis Sci. 2015 May;60(5):1482-9. doi: 10.1007/s10620-014-3453-3. Epub 2014 Nov 30. PMID: 25433921; PMCID: PMC4688457. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4688457/
Addition
(Chen, et al., 2018) Chen, Yi & Wang, Ningjian & Chen, Yingchao & Li, Qin & Han, Bing & Chen, Chi & Zhai, Hualing & Lu, Yingli. (2018). The association of non-alcoholic fatty liver disease with thyroid peroxidase and thyroglobulin antibody: A new insight from SPECT-China study. Autoimmunity. 51. 1-7. 10.1080/08916934.2018.1488968. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7460638/
Elevated levels of TPOab are associated with non-alcoholic fatty liver disease. (Chen, et al., 2018)
“The inflammatory pattern in NAFLD/NASH is usually represented by high TNF-α, interleukin-6 (IL6), and chemokine levels [146]. Along with an accumulation of hepatic FFAs and inflammation, malfunction of the mitochondria appears. The altered mitochondria, also called the “powerhouse” of the cell [147], leads to excessive oxidation and overflow of reactive oxygen species (ROS). These ROS products impair the activity of deiodinases [148], enhance lipid peroxidation, activation of the KCs, and of specific pro-inflammatory cytokines. Among them, the TNF-α and transforming growth factor β (TGF-β) activate hepatic satellite cells and endorse fibrosis [149,150].” (Chen, et al., 2018)
“One of the mechanisms possibly involved in the hypothyroidism-NAFLD pathogenesis could be represented by adipokine metabolism. Low circulating levels of thyroid hormones influence certain adipocytokines levels such as adiponectin or leptin. Adiponectin can enhance fatty acid oxidation and inhibit DNL through activation of AMP-activated protein kinase [158,159]. Hypothyroid subjects have altered adiponectin levels that can contribute to IR development. The modified adipocytokines registered have hepatotoxic properties, can promote oxygen radical release, and furnish liver inflammation and fibrosis [160,161]. Leptin is one hormone that is in charge of appetite modulation, can promote hepatic collagen synthesis hepatic IR, and is involved in hepatic fibrogenesis [162]. High levels of this hormone were found in patients with thyroid disfunction and seems to be correlated with TSH levels and with BMI [163]. Another adipokine studied in NAFLD and hypothyroidism is visfatin. In NAFLD, and especially in NASH subjects, low levels of visfatin have been described, however, an exact explanation behind this association is still unclear [164]. Recently, it was proposed that visfatin could be a promising serum biomarker for monitoring liver disease in younger obese population [165].” (Chen, et al., 2018)
“In a recent study published in 2019, thymoquinone (TQ) is used for restoring the liver structure to its initial state after induced hypothyroid NAFLD. TQ is the main constituent of Nigella salvia (NG), a medicinal plant representing the Ranunculaceae family [197]. NG is mostly used for its antioxidant and anti-inflammatory properties, but it also has an immunomodulatory effect [198]. […] Advanced fibrosis was detected through alpha-smooth muscle actin (α-SMA) staining [199]. A significant increase in the α-SMA reaction in the livers of the 6-propyl-2-thiouracil (PTU) treated group was observed mainly in the portal region associated with focal inflammatory response areas. The α-SMA index was restored to that of the control group after TQ administration. α-SMA staining was also used in the detection of activated hepatic stellate cells (HSCs) [200], which is a key element in hypothyroidism induced NAFLD.” (Chen, et al., 2018)
Addition
“Robust evidence is needed to delineate the nature behind NAFLD-hypothyroidism pathogenesis, how they influence each other, and if, in the near future, a program of detecting the thyroid function in NAFLD patients would be beneficial.” (Tanase, et al., 2020)
(Tanase, et al., 2020) Tanase DM, Gosav EM, Neculae E, Costea CF, Ciocoiu M, Hurjui LL, Tarniceriu CC, Floria M. Hypothyroidism-Induced Nonalcoholic Fatty Liver Disease (HIN): Mechanisms and Emerging Therapeutic Options. Int J Mol Sci. 2020 Aug 18;21(16):5927. doi: 10.3390/ijms21165927. PMID: 32824723; PMCID: PMC7460638. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7460638/
“Large-scale epidemiological studies provided evidence for a direct link between primary hypothyroidism and NAFLD by showing a higher prevalence of NAFLD in overt than in subclinical hypothyroidism…”
Abstract: “Nonalcoholic fatty liver disease (NAFLD) is a worldwide rising challenge because of hepatic, but also extrahepatic, complications. Thyroid hormones are master regulators of energy and lipid homeostasis, and the presence of abnormal thyroid function in NAFLD suggests pathogenic relationships. Specifically, persons with hypothyroidism feature dyslipidemia and lower hepatic β-oxidation, which favors accumulation of triglycerides and lipotoxins, insulin resistance, and subsequently de novo lipogenesis. Recent studies indicate that liver-specific thyroid hormone receptor β agonists are effective for the treatment of NAFLD, likely due to improved lipid homeostasis and mitochondrial respiration, which, in turn, may contribute to a reduced risk of NAFLD progression. Taken together, the possible coexistence of thyroid disease and NAFLD calls for increased awareness and optimized strategies for mutual screening and management.”
“The definition of NAFLD requires >5% of fat-loaded hepatocytes by histology or >5.6% triglyceride content by magnetic resonance imaging (MRI) or spectroscopy, in the absence of relevant alcohol abuse or other specific causes, including viral infections [1.]. NAFLD comprises of a wide spectrum of liver pathologies, ranging from simple steatosis, nonalcoholic steatohepatitis (NASH) to liver fibrosis (stages F1–F4), cirrhosis, and eventually hepatocellular carcinoma (HCC) [1., 2.]. Over the last few decades, NAFLD has become the most common liver disorder [1.], currently affecting about 25% of adults worldwide [3.]. Up to 80% of obese individuals have NAFLD, which has therefore been linked to the so-called metabolic syndrome (MetS) [1., 4., 5.]. Consequently, increased liver fat content is already present in about 60% of people with type 2 diabetes mellitus (T2DM) [4., 6.], soon after their diagnosis of diabetes [7.]. Hepatic fat content, however, differs among the recently proposed subtypes (endotypes) of T2DM, being particularly elevated in severe insulin-resistant diabetes (SIRD) [8.]. (Hatziagelaki, et al., 2022)
(Hatziagelaki, et al., 2022) Hatziagelaki E, Paschou SA, Schön M, Psaltopoulou T, Roden M. NAFLD and thyroid function: pathophysiological and therapeutic considerations. Trends Endocrinol Metab. 2022 Nov;33(11):755-768. doi: 10.1016/j.tem.2022.08.001. Epub 2022 Sep 26. PMID: 36171155. https://www.cell.com/trends/endocrinology-metabolism/fulltext/S1043-2760(22)00160-6
Creatine monohydrate has been found to be protective for treatment of NAFLD - non-alcoholic fatty liver - homocysteine levels were reduced, but it was not helpful or made alcoholic fatty liver disease worse. (Brave AI summary) Dosage used in sports nutrition is 3-5 grams per day. (Naderi, et al., 2016; Brave AI summary)
Elite sports nutrition gets into a level of detail that is not seen in standard nutrition counseling: “[Conclusion] Consumption of the supplements are usually suggested into 5 specific times, such as pre-exercise (nitrate, caffeine, sodium bicarbonate, carbohydrate and protein), during exercise (carbohydrate), post-exercise (creatine, carbohydrate, protein), meal time (β-alanine, creatine, sodium bicarbonate, nitrate, carbohydrate and protein), and before sleep (protein). In addition, the recommended dosing protocol for the supplements nitrate and β-alanine are fixed amounts irrespective of body weight, while dosing protocol for sodium bicarbonate, caffeine and creatine supplements are related to corrected body weight (mg/kg bw). Also, intake duration is suggested for creatine and β-alanine, being effective in chronic daily time < 2 weeks while caffeine, sodium bicarbonate are effective in acute daily time (1-3 hours). Plus, ingestion of nitrate supplement is required in both chronic daily time < 28 days and acute daily time (2- 2.5 h) prior exercise.” (Naderi, et al., 2016)
(Naderi, et al., 2016) Naderi A, de Oliveira EP, Ziegenfuss TN, Willems MT. Timing, Optimal Dose and Intake Duration of Dietary Supplements with Evidence-Based Use in Sports Nutrition. J Exerc Nutrition Biochem. 2016 Dec 31;20(4):1-12. doi: 10.20463/jenb.2016.0031. PMID: 28150472; PMCID: PMC5545206. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5545206/
(Casciola, et al., 2023) Casciola R, Leoni L, Cuffari B, Pecchini M, Menozzi R, Colecchia A, Ravaioli F. Creatine Supplementation to Improve Sarcopenia in Chronic Liver Disease: Facts and Perspectives. Nutrients. 2023 Feb 8;15(4):863. doi: 10.3390/nu15040863. PMID: 36839220; PMCID: PMC9958770. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9958770/
Abstract: “Creatine supplementation has been one of the most studied and useful ergogenic nutritional support for athletes to improve performance, strength, and muscular mass. Over time creatine has shown beneficial effects in several human disease conditions. This review aims to summarise the current evidence for creatine supplementation in advanced chronic liver disease and its complications, primarily in sarcopenic cirrhotic patients, because this condition is known to be associated with poor prognosis and outcomes. Although creatine supplementation in chronic liver disease seems to be barely investigated and not studied in human patients, its potential efficacy on chronic liver disease is indirectly highlighted in animal models of non-alcoholic fatty liver disease, bringing beneficial effects in the fatty liver. Similarly, encephalopathy and fatigue seem to have beneficial effects. Creatine supplementation has demonstrated effects in sarcopenia in the elderly with and without resistance training suggesting a potential role in improving this condition in patients with advanced chronic liver disease. Creatine supplementation could address several critical points of chronic liver disease and its complications. Further studies are needed to support the clinical burden of this hypothesis.” (Casciola, et al., 2023)
Creatine is involved in energy utilization - the exchange of phosphorus groups with ATP releasing energy from glucose or acetyl CoA in mitochondrial glycolysis. Creatine is needed in greater amounts in active tissues like muscles and the brain. Supplementing with it might be at a level of 2 to 5 grams per day up to 20 grams per day. It is is protein foods like meat and fish. We can make it internally also, making about half our need. Intake of one to two grams per day from the diet is typical. (Casciola, et al., 2023)
“Definition: Creatine is a guanidino compound naturally present in nature and mainly synthesised in the liver, kidneys, and pancreas [6]. It is known for its role in the intracellular storage of metabolic energy. Creatine is converted to phosphocreatine (PCr) by creatine kinase (CK), which catalyses the reversible transference of a phosphoryl group, allowing a rapid phosphate high energy bond exchange operated to generate adenosine triphosphate (ATP). This form of energy storage is particularly important in tissues with high and rapid energetic needs, such as muscles and the brain [1,6,7].
Creatine is present naturally in food, particularly in meat and fish; humans on a western diet get about one-half of their creatine from the diet and one-half by synthesis. From diet intake, the daily nutritional requirement averages 1–2 g of creatine [8]. The importance of endogenous synthesis of creatine is related to the physiological spontaneous and irreversible conversion to creatinine and, subsequently, urine loss. For example, in a 70-kg male in normal conditions, there is a loss of about 1–2 g of creatine [1,3,4,8,9].” (Casciola, et al., 2023)
Creatine supplementation might help prevent muscle loss (sarcopenia) in older people and it is used to enhance muscle development in athletes. It would likely help people with fatty liver conditions as it has shown benefits in animal-based trials, however human clinical trials are still a need. (Casciola, et al., 2023)
Section 5.1: “In our knowledge, only in rat models of NAFLD/NASH, the benefit of creatine supplementation has been demonstrated by decreasing homocysteine (Hcy) production in the liver, diminishing fat accumulation and bringing beneficial effects in the fatty liver [3]. Moreover, creatine supplementation can also prevent fatty liver, as shown in two studies [60,61] performed in rats fed with a high-fat diet and choline-deficient diet plus creatine compared to those without creatine. In humans, a study performed on 34 subjects showed how creatine monohydrate supplementation for 56 days (20 g/day for 5 days and then at 5 gr/day for 51 days) might significantly reduce blood lipids at 4 and 8 weeks (significant reductions in total plasma cholesterol by 6% and 5% respectively, triacylglycerols and very-low-density lipoprotein-C by 23% and 22% respectively) [53].
Fat accumulation and NAFLD/NASH progression are strictly associated with the shortage of methionine metabolism in the liver, leading to diminished availability of SAM, an elevation in Hcy levels and oxidative stress generation. Normally creatine biosynthesis uses SAM stores and also produces Hcy in the liver. In line, creatine supplementation is known to decrease the consumption of SAM and to reduce the Hcy production in the liver, leading to a decrease of triglycerides and fat synthesis and subsequent liver accumulation [52].” (Casciola, et al., 2023)






For the past 15 years I have watched reversals. I am not allowed to use the cure word. I am not allowed to speak about a specific medical condition. I can say... For $1 per day endogenous glutathione can be leveled up to normal. Truly safe and effective.
I’ve found creatine to be helpful.