VEGF, galactin-3, chimeric spike, and milk sugar.
My pomegranate research collided with a recent finding about the chimeric spike and with the heart attack and milk drinker's topic. Also, quercetin, fisetin, rhubarb, and being young helps.
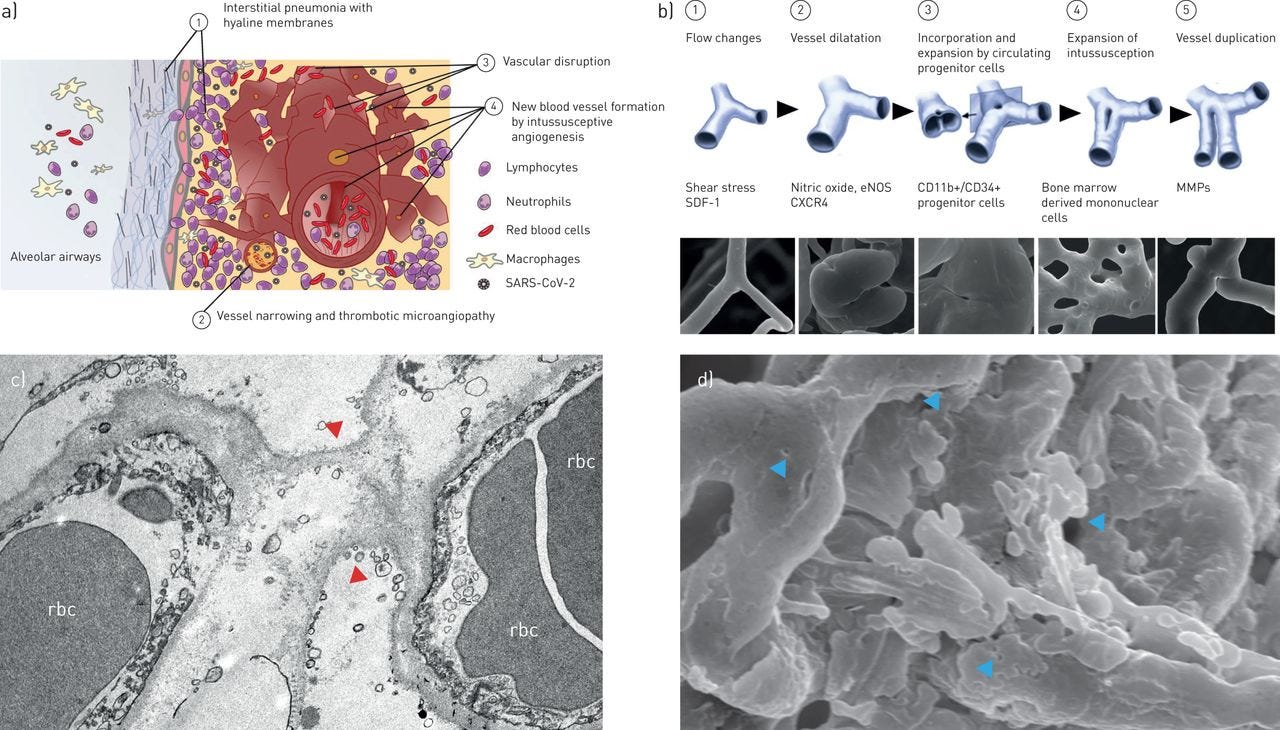
Angiogenesis is the formation of new blood vessels, which is great if they are needed, but not great if it is a cancerous tumor. And not great if it is lung tissue in a Covid patient. Doubling of blood vessels have been observed - intussusceptive angiogenesis.
*This post got long, too long for the email, click through and stick with it if interested in SPED topics - endothelial tissue is blood vessels. **Addition - several of the main links about galectin-3 and CoV were from a Twitter Thread and one reply to it, also the rhubarb link was via my Telegram Chat group. Thanks everyone for sharing info! The Tree of Knowledge needs many branches! I did add more links and connected some dotted lines.
Addition: The Too Long; Didn’t Read version.
The fiber content of the inner pith includes pectin which adds to the synergistic value as an inhibitor of galectin-3. Rhubarb pectin and other plant pectin are effective galectin-3 inhibitors and beneficial for fibrotic cardiovascular conditions. (Pozder Geb Gehlken, et al, 2021) The spike subunit S-1 has a similar sequence to galectin-3 which may be promoting angiogenesis. (Schroeder and Bieneman, 2022) Galectin-3 promotes angiogenesis by affecting the vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) control of angiogenesis. (Mehta and Granstein, 2019). Beta-lactose (milk sugar) and dominant-negative galectin-3 are also inhibitors. (Markowska, et al, 1981)
VEGF and its receptors are increased in myocarditis. (Li J, et al, 1996) Galectin-3 has a high predictive value as a marker for increased risk of re-hospitalization and mortality in heart failure patients. Change in level or maintenance also had predictive value and appeared in a dose related manner – a larger increase had a worse risk compared to smaller increase and a decrease was protective compared to maintaining an elevated level. (Chen et al., 2015, cited by Amin et al, 2017) Inhibiting Galectin-3 with GB1107 led to reduced IL-1, IL-6 and TNF-α levels in a spinal cord injury model. (Ren et al., 2019, cited by Caniglia, et al, 2020) Abnormal expression of galectins is seen in liver damage (Yang A, et al, 2021) and galectin-3 excess is associated with fibrotic kidney damage, while it is anti-inflammatory for an acute risk. (Chen and Kuo, 2016)
Galectin-3 has regulatory effects on apoptosis and disruption might lead to senescent cells. (Seyrek, et al, 2019) Senescent cells are a greater risk for elderly patients and quercetin and fisetin are two phytonutrients with anti-senolytic benefits. (Lynch, et al, 2021) Pomegranate peel has quercetin content. (Gullón, et al, 2020, Fig. 1)
Zinc also gets a mention, and α-Melanocyte-stimulating hormone (α-MSH), Beta-ionine and a good suntan.
More about α-MSH and weight control is included in the next post: α-MSH, regulator of appetite and weight and our bone matrix. (& we need Zinc too!) (substack.com) and Rhubarb is featured in the next one: Rhubarb deserves more than one line. (substack.com).
The post:
Galectin-3 is a carbohydrate-binding protein which promotes angiogenesis by mediating “the vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF)-mediated angiogenic response.” (Mehta and Granstein, 2019)
"Recent studies have shown that a carbohydrate-binding protein, galectin-3, is a novel pro-angiogenic molecule. The mechanism by which galectin-3 promotes angiogenesis remains unknown. We demonstrate here that galectin-3 is a mediator of vascular endothelial growth factor (VEGF)- and basic fibroblast growth factor (bFGF)-mediated angiogenic response. Angiogenesis assays revealed that galectin-3 inhibitors, β-lactose and dominant-negative galectin-3, reduce VEGF- and bFGF-mediated angiogenesis in vitro and that VEGF- and bFGF-mediated angiogenic response is reduced in galectin-3 knockdown cells and Gal3−/− animals." (Markowska, et al, 1981)
Ahem, beta-lactose is a form of milk sugar, a disaccharide of galactose and glucose. Since I avoid dairy products due to autoimmune or other food sensitivities, I will have to look for the milk free version of milk sugar as that might be more tolerable (?) for me - refined beta-lactose: (fishersci.com/beta-lactose-anhydrous-99-spectrum-chemical/18600939).
The dairy using heart study participants (this post: Too much calcium a heart attack risk? (substack.com)) may have been helping reduce angiogenesis risk associated with Acute Myocardial Infarction by inhibiting VEGF. (Li J, et al, 1996)
“In an adult heart, angiogenesis can occur in a number of pathological conditions, including atherosclerosis, hypertrophy, and infarction. […] In summary, acute myocardial infarction is accompanied by rapid and prolonged increase in expression of VEGF and its receptors with characteristic spatial and temporal kinetic. These findings suggest that the VEGF/VEGF receptor system plays an important role in the angiogenesis and stromal deposition associated with myocardial infarction.” (Li J, et al, 1996)
In the Covid19 patients, early outbreak article: (Ackermann, et al, 2020), the chimeric spike was likely increasing VEGF because it has domains that are similar - yet another lock the chimeric master key can pick.
“It is further known that the spike protein (S) of SARS-CoV-2 (as first reported for other β-coronaviruses) possesses a so-called galectin-fold within the N-terminal domain of the S1 subunit (S1-NTD). This fold (or pocket) shows structural homology nearly identical to that of human galectin-3 (Gal-3). In this respect, we have recently shown that Gal-3, when associated with epithelial cells or anchored to a solid phase matrix, facilitates the activation of innate immune cells, including basophils, DC, and monocytes. A synthesis of these findings prompted us to test whether segments of the SARS-CoV-2 spike protein might also activate innate immune cells in a manner similar to that observed in our Gal-3 studies. Indeed, by immobilizing S components onto microtiter wells, we show that only the S1 subunit (with the NTD) activates human monocytes to produce a near identical pattern of cytokines as those reported in COVID-19-related [cytokine release syndrome] CRS. In contrast, both the S1-CTD/RBD, which binds ACE2, and the S2 subunit (stalk), failed to mediate the same effect.” (Schroeder and Bieneman, 2022)
Another review team has information about medication galectin-3 inhibitors as a potential COVID19 treatment, including GB1107, TD139, and belapectin (also known as GR-MD-02).
“A study by Chen et al. (2015) has shown that Gal-3 inhibition simultaneously reduces the production of inflammatory cytokines such as IL-1 and IL-6 while also increasing the levels of the anti-inflammatory interleukin 10 (IL-10) in human dendritic cells. A reduction in IL-1, IL-6 and TNF-α levels upon treatment with the Gal-3 inhibitor GB1107 was also seen in an inflammatory model of spinal cord injury (Ren et al., 2019).” (Caniglia, et al, 2020)
Fusion inhibitors such as pomegranate peel would prevent free S1.
The fusion inhibitor potential of pomegranate peel would help by preventing the separation of the chimeric spike into the S1 and S2 subunits. The S1 is freed, and the S2 stays with the base, attached to a cell membrane (in injected people) or a viral membrane (in a real infection).
The galectin-3 look alike aspect of the S1 subunit may also be causing liver damage.
“Galectins play a regulatory role in liver diseases by binding their CRDs to the glycoconjugates expressed in the hepatocytes (9). Abnormal expression of the galectins may be related to the development of hepatitis and liver fibrosis/cirrhosis and the progression of HCC (10).” (Yang A, et al, 2021)
. . . and kidney damage.
“Galectin-3 has been reported to promote nephrogenesis and to be strongly expressed in the ureteric bud and its derivatives. Furthermore, an increased concentration of galectin-3 has been reported to be associated with fibrosis of the kidneys. Furthermore, elevated levels of plasma galectin-3 are associated with increased risks of rapid renal function decline and of incident chronic kidney disease (Figure 2). Galectin-3 has also been reported to play a role in ameliorating inflammation, with an increased concentration in response to ischemia and nephrotoxic acute kidney injury [19]. In addition, it has also been reported to prevent chronic tubular injury by reducing apoptosis and fibrosis, and increasing matrix remodeling [23]. Its reno-protective properties are evidenced by the rapid development of diabetic nephropathy in galectin-3–/– mice [24]. However, in persistent or repetitive tissue injury, it may modulate the transition to chronic inflammation and fibrosis [9].” (Chen and Kuo, 2016)
. . . and mortality from heart failure and other heart damage risks.
“Numerous studies have shown galectin-3 as a novel prognostic biomarker with high predictive value for cardiovascular mortality and re-hospitalization in [heart failure] HF patients.” (Amin et al, 2017)
One of the studies, Chen et al, mentioned by (Amin et al, 2017), found that patients with a 15% or greater change in galectin-3 levels were significantly more likely to improve, or worsen, depending on the direction of change or maintaining an elevated level. Reduction in galectin-3 was linked to lower risk while compared to an increase or a continual elevation in the level.
“Additionally, meta-analysis by Chen et al demonstrated that from pooled analysis of 9 studies, every 1% increase of galectin-3 level was also followed by 28% increased risk of all-cause mortality (HR 1.28 95% CI 1.10–1.48) with significant heterogeneity (I2 = 82.1%) [22].” (Amin et al, 2017)
Addition via the rhubarb link on Telegram:
“Conclusion: The galectin-3 inhibitor natural rhubarb pectin has a superior inhibitory capacity over established pectins, substantially attenuates cardiac fibrosis, and preserves cardiac function in vivo. Bioactive pectins are natural sources of galectin-3 inhibitors and may be helpful in the prevention of heart failure or other diseases characterized by fibrosis.” (Pozder Geb Gehlken, et al, 2021)
Galectins in apoptosis.
Galectins are also involved in the regulation of apoptosis - controlled cell death of damaged, infected, or old cells. (Seyrek, et al, 2019) Disruption of apoptosis can lead to senescent cells that are dysfunctional but not being removed as they should in normal health, or a more explosive type of cell death (pyroptosis) that leaks inflammatory contents of the cell into the surrounding tissue - leading to more inflammation. Increased senescence has been noted in Covid19. Senolytic medications or phytonutrients might help. Elderly patients would be more at risk for developing senescent cells. (Lynch, et al, 2021) We need a functioning detoxification and immune system to quickly remove cellular debris before it causes more damage to nearby cells.
“Viral infection via SARS-CoV-2 triggers a dysregulated reaction referred to as the “cytokine storm” during the early stage of infection. During the cytokine storm, an array of inflammatory cytokines and chemokines such as IL-6, IL-8, IL-12, IL-1β, CXCL-10, CCL-2, IFN-γ, and TNF-α are emitted. Several of these inflammatory molecules have the capacity to instigate “paracrine” senescence via a persistent cytokine signaling [112,167,168,172,173]. Infection via the SARS-CoV-2 virus is thought to instigate inflammatory cell death otherwise known as pyroptosis [174].”
“These findings demonstrate the hypothetical rationale behind the therapeutic potential of senolytics, particularly in aged patients who have a higher burden of senescence, to interrupt the initial triggered immune cascade, cytokine storm and hyperinflammation evident in severe cases of SARS-CoV-2 infection. Indeed, Quercetin and Fisetin, which have excellent safety profiles thus serve as attractive candidates, are currently being investigated in COVID-19 trials as potential senolytic compounds for early intervention [272,273,274].” (Lynch, et al, 2021)
Fisetin- is a "flavonoid found in...(strawberries, apples, mangoes, persimmons, kiwis, & grapes), vegetables (tomatoes, onions, and cucumbers), nuts, & wine that has shown strong anti-inflammatory, anti-oxidant, anti-tumorigenic, anti-invasive, anti-angiogenic" properties. (13)
Quercetin - is a flavanol, a type of flavonoid, found in onions, garlic, green leafy veg, citrus peel, figs, and is a focus of several recent posts: Citrus Fig jam: (14), Hesperidin & quercetin content in citrus peel: (15), Decongestant properties of hesperidin/citrus peel: (16). Quercetin represents a large group of similar chemicals and is fairly common in small amounts in many fruits, vegetables and leafy herbs. It is found primarily in the leaves or edible peels, including citrus and pomegranate peels. (jenniferdepew.com/phytonutrients)
What else might help besides milk sugar and senolytics such as quercetin and fisetin?
Rhubarb and other pectin rich foods (apples, pomegranate, inner peel) might also help as galectin-3 inhibitors. (Pozder Geb Gehlken, et al, 2021) Galectin-3 inhibitors as a Covid19 treatment are discussed (Caniglia, et al, 2020)
And α-Melanocyte-stimulating hormone (α-MSH) can help inhibit angiogenesis by attenuating VEGF signaling.
"It has recently been shown that α-MSH inhibits angiogenesis by attenuating vascular endothelial growth factor (VEGF) signaling in endothelial cells [19]." (Mehta and Granstein, 2019)
. . . and that is probably why too much sun or astronauts being irradiated can cause worsening of inflammatory symptoms. UV radiation or infection can increase inflammatory signals, one of which is a precursor for the anti-inflammatory (α-MSH) - a built in negative feedback control that could help prevent escalating hyperinflammation.
“α-Melanocyte-stimulating hormone (α-MSH) is a tridecapeptide that is generated from the precursor hormone proopiomelanocortin via proteolytic cleavage [8]. Proopiomelanocortin generation is controlled by mediators such as corticotrophin-releasing hormone, exogenous noxious stimuli (ultraviolet irradiation, microbial agents), and pro-inflammatory cytokines (interleukin [IL]-1, tumor necrosis factor [TNF]-α as well as others) [9]. Proopiomelanocortin is produced by many cells in the skin including keratinocytes, Langerhans cells, melanocytes, and endothelial cells; after production it is cleaved to form adrenocorticotrophin hormone which upon proteolytic cleavage forms α-MSH [10].” (Mehta and Granstein, 2019)
What does α-MSH normally do? Promote a good suntan - more melanin production.
You know what else helps promote a good suntan (more melanin production)? You’ll never guess - probably - the fragrance of spring flowers - the terpene like carotenoid β-ionone helps tell our melanocytes to make more melanin when it is spring flower season. Mother Nature has got our back - we just have to stop to smell the flowers.
Excerpts from a podcast series (transcript with references, link to the audio): The aromatic chemical involved in the scent of violets is β-ionone. It is chemically similar to terpenes but is found with carotenoids - the vitamin A group. Beta-ionone is used in the synthesis of vitamin A, vitamin E and vitamin K, (8) and in perfumes. (9) Greater concentrations of the chemical smell like cedar wood and more dilute amounts have the floral scent characteristic of violets. (10) It is so potent of a fragrance that only a very dilute amount is typically present in a flower or fruits like blackberries, peaches, and apricots. (8)
Odd trivia - we have the odor receptor for beta-ionone in the retina of our eyes and it stimulates growth of the retinal pigment epithelial cells. (17) Why would we have an odor receptor outside of our nose? It is a chemical receptor, for a chemical that happens to be fragrant. We have a variety of receptors throughout our body for taste, odor, light. It’s helping inform about the environment probably within that cellular small zone. (25)
More odd trivia - the beta ionone odor receptors are also in our skin, in the melanocytes which make the pigment of skin color and the darker tones from a suntan. More needs to be learned about the function of the beta-ionone odor receptors within the light sensitive skin cells. (21) There seems to be a role for them in stimulating melanin production, the dark pigment of skin color, and also nerve growth and it may be protective against the overgrowth of skin cancer cells. So, use of beta-ionone in medications against skin cancer, or as a preventative in suntan lotions may be a future role for the fragrant chemical. (22)
*Retinoids seem involved - but I am done for now. ;-)
Don’t forget zinc!
***Important point about odor and taste receptors, where-ever they are found in the body, they needed zinc in order for the protein to be transcribed from the gene for the receptors. Elderly people would be much more at risk for any dysfunction related to odor or taste receptors because their need for zinc is elevated. Zinc is also needed for differentiation of immature immune cells to functional ones that can help fight an infection or clean up cellular debris or aged or defective cells.
Good rule of thumb - anytime quercetin is recommended keep zinc in mind too. They are a team. Thanks Dr. Zelenko (rest in peace) for your work on spreading the good news about zinc ionophores!
Disclaimer: This information is provided for educational purposes within the guidelines of fair use. While I am a Registered Dietitian this information is not intended to provide individual health guidance. Please see a health professional for individual health care purposes.
Reference List
(Ackermann, et al, 2020) Ackermann M, Mentzer SJ, Kolb M, Jonigk D. Inflammation and intussusceptive angiogenesis in COVID-19: everything in and out of flow. Eur Respir J. 2020 Nov 12;56(5):2003147. doi: 10.1183/13993003.03147-2020. PMID: 33008942; PMCID: PMC7530910. https://erj.ersjournals.com/content/56/5/2003147.long#F1 Figure 1 https://erj.ersjournals.com/content/erj/56/5/2003147/F1.large.jpg
(Amin et al, 2017) Amin HZ, Amin LZ, Wijaya IP. Galectin-3: a novel biomarker for the prognosis of heart failure. Clujul Med. 2017;90(2):129-132. doi: 10.15386/cjmed-751. Epub 2017 Apr 25. PMID: 28559694; PMCID: PMC5433562. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5433562/
(Caniglia, et al, 2020) Caniglia JL, Guda MR, Asuthkar S, Tsung AJ, Velpula KK. A potential role for Galectin-3 inhibitors in the treatment of COVID-19. PeerJ. 2020 Jun 15;8:e9392. doi: 10.7717/peerj.9392. PMID: 32587806; PMCID: PMC7301894. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7301894/
(Chen and Kuo, 2016) Chen SC, Kuo PL. The Role of Galectin-3 in the Kidneys. Int J Mol Sci. 2016 Apr 14;17(4):565. doi: 10.3390/ijms17040565. PMID: 27089335; PMCID: PMC4849021. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4849021/
(Gullón, et al, 2020, Fig. 1) Gullón P, Astray G, Gullón B, Tomasevic I, Lorenzo JM. Pomegranate Peel as Suitable Source of High-Added Value Bioactives: Tailored Functionalized Meat Products. Molecules. 2020 Jun 21;25(12):2859. doi: 10.3390/molecules25122859. PMID: 32575814; PMCID: PMC7355679. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7355679/ (Accessed: 3 November 2022)
(Li J, et al, 1996) Li J, Brown LF, Hibberd MG, Grossman JD, Morgan JP, Simons M. VEGF, flk-1, and flt-1 expression in a rat myocardial infarction model of angiogenesis. Am J Physiol. 1996 May;270(5 Pt 2):H1803-11. doi: 10.1152/ajpheart.1996.270.5.H1803. PMID: 8928889. https://pubmed.ncbi.nlm.nih.gov/8928889/
(Lynch, et al, 2021) Lynch SM, Guo G, Gibson DS, Bjourson AJ, Rai TS. Role of Senescence and Aging in SARS-CoV-2 Infection and COVID-19 Disease. Cells. 2021 Nov 30;10(12):3367. doi: 10.3390/cells10123367. PMID: 34943875; PMCID: PMC8699414. https://pubmed.ncbi.nlm.nih.gov/34943875/
(Markowska, et al, 1981) Markowska AI, Liu FT, Panjwani N. Galectin-3 is an important mediator of VEGF- and bFGF-mediated angiogenic response. J Exp Med. 2010 Aug 30;207(9):1981-93. doi: 10.1084/jem.20090121. Epub 2010 Aug 16. PMID: 20713592; PMCID: PMC2931172. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2931172/
(Mehta and Granstein, 2019) Mehta D, Granstein R, D: Immunoregulatory Effects of Neuropeptides on Endothelial Cells: Relevance to Dermatological Disorders. Dermatology 2019;235:175-186. doi: 10.1159/000496538 https://www.karger.com/Article/Fulltext/496538
(Pozder Geb Gehlken, et al, 2021) Pozder Geb Gehlken C, Rogier van der Velde A, Meijers WC, Silljé HHW, Muntendam P, Dokter MM, van Gilst WH, Schols HA, de Boer RA. Pectins from various sources inhibit galectin-3-related cardiac fibrosis. Curr Res Transl Med. 2022 Jan;70(1):103321. doi: 10.1016/j.retram.2021.103321. Epub 2021 Nov 23. PMID: 34826684. https://linkinghub.elsevier.com/retrieve/pii/S2452-3186(21)00047-7
(Ren et al., 2019) Ren Z, Liang W, Sheng J, Xun C, Xu T, Cao R, Sheng W. (2019) Gal-3 is a potential biomarker for spinal cord injury and Gal-3 deficiency attenuates neuroinflammation through ROS/TXNIP/NLRP3 signaling pathway. Bioscience Reports. 2019;39(12):BSR20192368. doi: 10.1042/BSR20192368. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6923351/ (Accessed: 3 November 2022)
(Schroeder and Bieneman, 2022) Schroeder JT., Bieneman AP. The S1 Subunit of the SARS-CoV-2 Spike Protein Activates Human Monocytes to Produce Cytokines Linked to COVID-19: Relevance to Galectin-3, Frontiers in Immunology, 13;2022 DOI=10.3389/fimmu.2022.831763, ISSN=1664-3224 https://www.frontiersin.org/articles/10.3389/fimmu.2022.831763/full via https://twitter.com/TurdFur58439807/status/1568140878534909955?s=20
(Seyrek, et al, 2019) Seyrek, K., Richter, M., & Lavrik, I.N. (2019). Decoding the sweet regulation of apoptosis: the role of glycosylation and galectins in apoptotic signaling pathways. Cell Death & Differentiation, 26, 981-993. https://www.semanticscholar.org/paper/Decoding-the-sweet-regulation-of-apoptosis%3A-the-of-Seyrek-Richter/ecfd361094b4bee39cfc884cf9b6461b81e7ea02
(Yang A, et al, 2021) Yang A, Shixue X, Yiting L, et al, Role of Galectins in the Liver Diseases: A Systematic Review and Meta-Analysis, Frontiers in Medicine, 8;2021 DOI=10.3389/fmed.2021.744518, ISSN=2296-858X https://www.frontiersin.org/articles/10.3389/fmed.2021.744518




Wow! This is great research. Thank you for your doggedness. In 2004 Ralph Baric wrote that “blocking interferon-1 was a tactic used by pathogenic RNA viruses”, but beta-coronaviruses was not one of them until SARS-2 arrived in December 2019 with the Furin clevage and its ability to get into the nucleus and disable interferon and activate aberrant growth factors causing widespread fibrosis as described in your excellent article. Quercetin is a zinc ionophore that facilitates zinc’s ability to block SARS-2 activation of the Stat-3 pathway. Also proteolytic enzymes like Lumbrokinase help break down fibrin formation and fasting activates apoptosis of senescent cells.
Great work, please continue to shine a light into dark corners.