How can vitamin A deficiency and excess cause the same symptoms?
That is believed to be what happens, BUT, the symptoms might really be caused by zinc, protein and taurine deficiency instead. Post too long, and should be a document but isn't yet.
*Addition - Vitamin A is helpful for fighting infection and low levels are associated with more severe illness or risk of infection for measles. But, it can seem low in blood tests while really there is plenty in the liver. It needs transport proteins to be made in the liver and then it is sent out to help the body have an immune response if called into action. Some nutrients are also important for preventing retinoid excess - too much activated vitamin A, because some can help hold it in an inactive form, reversibly - ready for action if needed and others can help contain it for removal, an irreversible removal from action. That is needed to help prevent an excess from accumulating.
Retinoid Toxicity and vitamin A deficiency can "cause" the same symptoms - or is it really something else causing those symptoms?
tl;dr - lack of zinc, taurine, niacin, and/or phosphatidylethanolamine, and lack of protein in general, may be the real underlying cause of "Vitamin A deficiency" or "Retinoid Toxicity" - however, if the problem of Retinoid Toxicity does exist - add those things, but also it would help to cut down or cut out vitamin A and carotenoids because the liver accumulation can last quite a while, so reversing symptoms can improve initially but then take a while to totally normalize.
Dr. Garrett Smith, also known as the Nutrition Detective, has shared a lot of information on his site and on X.com about Retinoid Toxicity as a major underlying cause of many “conditions”. Dr. Smith has found that a lack of sufficient protein - a macronutrient, and more specifically a lack of taurine rich muscle meat, is a causal factor of the “vitamin A symptoms” and lack of zinc is likely involved in many cases too.
Symptoms of Retinoid Toxicity and symptoms of Histamine Excess are very similar too, and I have both listed in a Table from my Pomegranate paper: Table 1. Symptoms of Histamine and Retinoid Toxicity (Dropbox) Activated retinoids can trigger degranulation of allergy mast cells which releases histamine - so the excess of activated vitamin A is also an underlying cause of histamine excess. I wrote two different versions of that paper, and the first version was more focused on the histamine issue: Version 1. Pomegranate, TRP channels, nociceptive pain and endocannabinoids (transcendingsquare.com)
Thread: (x.com/NutriDetect); Unrolled Thread Reader version: x.com/UnrollHelper) <that link didn’t work on my laptop.
Sign up at Dr. Smith’s site for a free newsletter/course: Madness of Modern Nutrition Course, (nutritiondetective.com)
Muscle meats (and shellfish) are good sources of zinc compared to many other foods. Pumpkin seeds are a pretty good source of zinc among vegan or vegetarian foods. Other beans, seeds and nuts have some too. Dairy products are not a very good source of taurine or zinc. Vegetarians or ‘meat eaters’ who get most of their protein from cheese and milk products may end up with symptoms of “Retinoid Toxicity” - and reducing vitamin A and carotenoids would likely help improve the negative symptoms, BUT, increasing taurine and zinc and total protein intake might also be needed before improvement will occur - and then the vitamin A/carotenoid foods might also be less of a problem.
Lack of iodine and selenium and gluten sensitivity/molecular mimicry leading to autoimmune hypothyroid problems might also be involved, as the thyroid hormone and the thyroid receptor have some co-regulatory control over retinoid metabolism and gene transcription by the retinoid receptors may also involve co-regulation by the thyroid receptor or vitamin D receptors. Zinc finger proteins also have regulatory control over gene transcription and lack of it can affect the function of the Vit D, A, or thyroid receptors.
Both “vitamin A” and “vitamin D” are really hormones which we can make for ourselves if we have the proper sunshine and precursor nutrients. That makes them both potentially dangerous compared to other “vitamins” as an excess may cause a variety of inflammatory proteins to be made. And if active vitamin D is in excess, then hypercalcemia is causal of negative symptoms and can become dangerous as well as promoting irritability leading to worse anger or even “Roid Rage”.
Vitamin A in the active form is too dangerous to be allowed to travel freely. It needs Retinal Binding Protein, (RBP) to act as a carrier protein. Also called RBP4, the transport protein carries inactive retinol or activated forms of Retinoic Acid. (Brave AI summary; Kanai, et al., 1968; Muenzner, et al., 2013)
Serum levels of RBP4 tend to be elevated in more severe chronic illness (examples included later impact kidney, heart, brain, and psoriasis) but may also be very low or within normal ranges. Lack of protein and other factors may be involved in a fragile patient having low levels. We wouldn’t know what the RBP4 is carrying, or if elevated levels might represent activated retinoids or inactive retinol. So, we wouldn’t know if Retinoid toxicity, excess activated vit. A, was present based only on the RBP4 lab test. Referring to the ‘Symptoms of Retinoid Toxicity’ list might add clarity - if it looks and quacks like a duck, it might be a duck. See: Table 1. Symptoms of Histamine and Retinoid Toxicity (Dropbox)
Zinc
Zinc seems to be needed for RBP to be made - a gene transcription role for zinc finger proteins. Zinc is also used in the enzyme, retinol dehydrogenase (RDH), (Smith, 1980), that converts the alcohol form of vitamin A (retinol) into the aldehyde form retinal or ‘retinaldehyde’. Short-chain dehydrogenase/reductases (SDR) can also do that conversion. Retinaldehyde can then be activated to Retinoic acid by retinaldehyde dehydrogenase (RALDH) or cytochrome P450 enzymes (CYP). (Kedishvili, 2016) (Brave AI summary) *Aside, glyphosate may be interfering with CYP enzyme function which would impact vitamin D metabolism too.
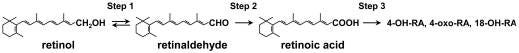
Taurine comes into play, in prevention of Retinoid Toxicity, because it can bind with retinaldehyde, in a reversible complex. Phosphatidylethanolamine (PE) can also sequester retinaldehyde with an additional step needed by NADPH to reduce it back to retinol (a two-way conversion being possible for maintaining retinol/retinoid balance). (Kim, et al., 2022) (Brave AI summary re my checking on the meaning of ‘reduced by NADPH’) Therefore… being low in cannabinoids or dysfunctional in their metabolism, or being low in niacin, might also add to a person’s risk for Retinoid Toxicity. Hmmm, that is very interesting.
“To facilitate the movement of retinoids through the visual cycle and to limit nonspecific chemical reaction, multiple mechanisms are utilized to handle these molecules when not contained within the binding pocket of opsin.
>Vitamin A aldehyde is sequestered by reversible Schiff base formation with phosphatidylethanolamine (PE) and subsequently undergoes NADPH-dependent reduction.” […]
>“Mechanisms for sequestration of retinoid include the formation of a reversible Schiff base between retinaldehyde and taurine (A1-taurine, A1T), the most abundant amino acid in photoreceptor cells.” (Kim, et al., 2022)
*More on NADPH/niacin and its role in Retinoid Toxicity risk follows the zinc info.
Zinc is used in approximately 300 enzymes and the gene transcription function of zinc finger proteins impacts our ability to make a variety of receptors and other proteins.
“Nuclear hormone receptors are an important family of regulatory proteins that includes the receptors for the hormones, retinoids, vitamin D, thyroid hormone, sex hormones (estrogens, progestins, and androgens), aldosterone, and glucocorticoids. These proteins contain separate regions for hormone binding, DNA binding, and for transcriptional activation. The DNA-binding domains contain two zinc atoms, which are each held in place by four cysteine residues.”
TRANSCRIPTION FACTORS | Overview, I.M. Adcock, ... G. Caramori, in Encyclopedia of Respiratory Medicine, 2006; via (ScienceDirect/Zinc finger proteins)
Zinc finger proteins are needed by keratinocytes. (Cassandri, et al., 2017) Zinc is needed for gene transcription in varied ways (Morris and Levenson, 2013) and we need to eat enough protein in general to make the many types of proteins that we need to produce each day.
NADPH - aka the role of lack of niacin in risk of Retinoid Toxicity
This helps clarify why starting high dose niacin helped me so much when I was also eating a lot of carotenoids and symptomatic - chapped lips with cracks at the corners of the mouth is characteristic of Retinoid Toxicity and I had other symptoms of histamine excess.
Retinoid-NADPH Toxic Interplay - Brave AI
Retinoids, derivatives of vitamin A, are used in the treatment of various skin conditions, including acne, psoriasis, and dermatitis. However, they can also cause toxicity, particularly when taken in excess or for prolonged periods. NADPH (Nicotinamide adenine dinucleotide phosphate) plays a crucial role in retinoid metabolism and toxicity.
Retinoid Toxicity
Retinoid toxicity can manifest in various ways, including:
Gastrointestinal symptoms: nausea, vomiting, diarrhea
Hepatotoxicity: liver damage, elevated liver enzymes
Bone toxicity: bone pain, osteoporosis
Neurotoxicity: headache, dizziness, blurred vision
Teratogenic effects: birth defects
NADPH’s Role in Retinoid Metabolism
NADPH is a reducing agent that plays a vital role in the metabolism of retinoids. It is involved in the reduction of all-trans-retinaldehyde to all-trans-retinol, a process catalyzed by retinol dehydrogenases (RDHs). This reaction is essential for maintaining retinoid homeostasis.
Implications of NADPH Deficiency
Deficiencies in NADPH or RDHs can lead to:
Accumulation of toxic all-trans-retinaldehyde
Inhibition of retinol production
Enhanced oxidative stress
Increased susceptibility to retinoid toxicity
Clinical Relevance
Understanding the interplay between retinoids and NADPH is crucial for optimizing retinoid therapy and minimizing toxicity. Clinicians should be aware of the potential risks of retinoid toxicity and monitor patients for signs of liver damage, bone toxicity, and other adverse effects.
Key Takeaways
Retinoids can cause toxicity, particularly when taken in excess or for prolonged periods.
NADPH plays a crucial role in retinoid metabolism, reducing all-trans-retinaldehyde to all-trans-retinol.
Deficiencies in NADPH or RDHs can lead to retinoid toxicity and oxidative stress.
Clinicians should monitor patients for signs of retinoid toxicity and adjust therapy accordingly.
Follow up
What are the specific mechanisms by which NADPH deficiency contributes to retinoid toxicity?
How do retinoid formulations (e.g., topical vs. oral) affect NADPH-mediated metabolism and toxicity?
pubmed.ncbi.nlm.nih.gov, Liver toxicity of retinoid therapy - PubMed
sciencedirect.com, Toxicology, carcinogenicity, and teratogenicity of some orally administered retinoids - ScienceDirect
nature.com, NAD+ metabolism, stemness, the immune response, and cancer | Signal Transduction and Targeted Therapy
sciencedirect.com, Retinol Intoxication - an overview | ScienceDirect Topics
ncbi.nlm.nih.gov, Retinol dehydrogenase 11 is essential for the maintenance of retinol homeostasis in liver and testis in mice - PMC
sciencedirect.com, All-Trans-Retinal - an overview, ScienceDirect Topics
More depth on Retinol Binding Protein, and potential use of the serum lab value as an indicator of disease risk.
RBP - Retinol Binding Protein as a lab indicator of elevated retinoids - but we don’t know if it is inactive retinol or activated retinoids. Chronic illness risk is associated with RBP but levels during the same chronic illnesses can also be near zero which could indicate a lack of protein or zinc for making RBP. Severity of disease for various conditions has been linked to higher levels of RBP which would suggest that it is activated retinoids being transported - they promote an immune response which is needed to fight infection but might not be needed based only on whether you had a vitamin A rich meal last night.
Retinol Binding Protein is transport for activated vitamin A.
“The single known function of this protein [RBP] is to transport retinol from the hepatic stores to target tissues.” (Quadro, et al., 2003) *More has been learned since 2003 about other functions of RBP, mentioned later.
We need to eat adequate amounts of protein and other nutrients in order to make new proteins within our bodies. Adults in Bangladesh with lower BMI, malnourished category, had lower RBP4 and that was associated with worse gut health parameters. (Fahim, et al., 2022)
Some people may lose more proteins in urine from poor kidney function.
Elevated RBP in urinary losses is seen in chronic kidney disease. RBP levels in urine could be used as a lab biomarker except for availability of the test within a patient care clinic. It is used in research.
Clinical use
Retinol Binding Protein testing is used by clinicians to assess renal tubular injury or dysfunction and screening for other tubular abnormalities. The test can also be used to detect chronic asymptomatic renal tubular dysfunction. (southtees.nhs.uk)
*But this urinary RBP lab test may not be readily available outside of research labs.
Labcorp does list RBP for blood serum levels: “Retinol-binding Protein (RbP), TEST: 123060, Test number copied CPT: 83883,” “Responsible for binding and transporting retinol (vitamin A).” (labcorp.com). That test could show if the liver was producing elevated levels of RBP which suggests elevated activated retinoids also.
“Serum concentrations reflect the synthesis capacity of the liver and may indicate early malnutrition, acute and chronic hepatic disease, advanced chronic renal insufficiency, and cystic fibrosis. Assess nephritic syndrome and protein-losing enteropathy. Due to the short half-life of approximately 12 hours, RBP may be suitable for monitoring the nutritional status and efficacy of parenteral nutrition.1” (labcorp.com)
Retinoid toxicity may occur due to Retinoid metabolism changes, or liver injury.
Overactivation of vitamin A to Retinoic acid can occur due to gene changes in the liver enzyme which might occur from certain infections (EBV and possibly SARS-CoV2). The growth of EBV (and possibly SARS2) is aided by the increase in Retinoic Acid, but the overactivation of retinoids and increased production and release of the transport protein leads to Retinoid excess for the person who had been sick with Epstein Barr Virus sometime in their past, (*highschool for me). Liver injury and possibly drug injury/akathisia may involve an ongoing excess of active retinoids. Serum RBP approximately shows circulating vitamin A levels but not stored liver levels, that can be elevated while serum levels are low - if protein is inadequate or zinc or taurine.
Elevated RBP4 is associated with Metabolic Syndrome, higher BMI and blood lipids, and increased insulin resistance.
“RBP4 levels were higher in male and Beijing residents, compared with female and Shanghai participants (both P < 0.001). RBP4 levels were associated positively with body mass index, waist circumference, triglycerides, total and low-density lipoprotein cholesterol, blood pressure, fasting insulin, and homeostatic model assessment of insulin resistance and negatively with high-density lipoprotein cholesterol and adiponectin (all P < 0.001). In the highest RBP4 quartile, the MetS risk was significantly higher (odds ratio 2.58; 95% confidence interval 2.08–3.20) than in the lowest quartile after adjustment for potential confounders.” (Qi, et al., 2007)
RBP4 is also considered an adipokine, which is inflammatory and cytokine-like but made by adipose (fat) cells. Levels of RBP4 have been associated with insulin resistance, inflammatory chronic illness and obesity. Type 2 diabetes, Metabolic Syndrome and cardiovascular disease is linked to elevated RBP4 - which means that those chronic issues are also linked to elevated retinoids…to vitamin A and carotenoids.
This is mind-blowing to me, because I swore by carrots as a superfood to eat every day, but I was making myself sicker - and more overweight apparently. My other underlying risk factors would have included low zinc, niacin and endocannabinoids though and possibly low taurine.
“Retinol binding protein 4 (RBP4), previously called retinol binding protein (RBP), is considered a specific carrier of retinol in the blood. It is also an adipokine that has been implicated in the pathophysiology of insulin resistance. RBP4 seems to be correlated with cardiometabolic markers in inflammatory chronic diseases, including obesity, type 2 diabetes, metabolic syndrome, and cardiovascular diseases (CVDs). It has recently been suggested that inflammation produced by RBP4 induces insulin resistance and CVD.” […] “Retinol-binding protein 4 (RBP4)8, also known as retinol binding protein (RBP), is a plasma retinol transporter that carries retinol from the liver to the periphery, and very little plasma RBP4 originates from adipose tissue (4).” (Zabetian-Targhi, et al., 2015)
I could collect a lot more articles on the topic of elevated RBP4 and Cardiovascular disease or Coronary Heart Disease (CVD/CHD):
A 16-year study of nurses: “In this cohort of women, higher circulating full-length and total RBP4 levels were associated with increased risk of [Coronary Heart Disease] CHD in a time-dependent fashion.” (Sun, et al., 2013)
Higher levels of serum RBP4 was associated with more “major adverse cardiovascular events (MACEs) in Chinese patients with stable [Coronary Artery Disease] CAD.” (Ke, et al., 2022)
What is a higher level of RBP4? The upper 3 quartiles in the study by Ke, et al, 2022, all had worse risk: “The mean level of serum RBP4 in all patients was 35.8 ± 11.7 μg/ml.” (Ke, et al., 2022) Math - that gives us a range of 24.1 to 47.5 μg/ml.
Labcorp “Normal range” for RBP: “0 to 12 y - 1.2−4.6; 13 to 60 y - 1.6−6.1; 61 to 80 y - 1.8−7.3; >80 y - 1.6−6.1 mg/dL” (Labcorp/RBP)
How many milligram/deciliter in 1 microgram/milliliter? The answer is 0.1. (convertunits.com) - 24.1 micrograms per milliliter would be 2.41 mg/dL if the search result is accurate, which means those people who already had CAD, also had RBP that was considered within the normal range. The top three quartiles were found to have the highest risk to have a major adverse event. Range: 241 to 475 mg/dL - the lower quartile would have been between ~ 2.41-2.995 mg/dL. (Ke, et al., 2022) “Normal” for a 65-year-old would have had a level between 1.8-7.3 mg/dL per Labcorp/RBP, (18-73 μg/ml) so even the 47.5 μg/ml (4.75 mg/dL) would be considered a normal level.
“Normal” lab value ranges are often simply set at the range that is commonly found in a population, which might be a patient population for a lab that mostly sees patients. In the study by Ke, et al., 2022, “normal” includes having a higher risk for major cardiovascular adverse events. Lower risk for heart disease patients would have a narrower range ~ 1.8-2.9 mg/dL RBP4.
…and News to Know childhood RBP has been linked to Metabolic Syndrome (MetS or MS) later in life.
Having elevated levels of RBP4 (*read as elevated retinoids/activated vitamin A) during childhood was associated with Metabolic Syndrome and insulin resistance ten years later (in a childhood study that took place in Beijing) — independent of pediatric obesity. The children with elevated RBP4 may have been of average weight. And levels of leptin and adiponectin were also measured and correlated with the RBP4 pattern of risk prediction. Previous pediatric studies had shown that weight loss helped improve the RBP4 and triglyceride levels and reduce insulin resistance. ([18, 19] Li, et al., 2018)
Weight loss helped improve RBP levels and Metabolic Syndrome in other pediatric studies on RBP4. ([18, 19],Li, et al., 2018)
Background: “While liver is the primary source of circulating RBP4, adipocytes become an important secondary source in insulin resistant states [6]. Elevations in RBP4 will induce adipose tissue inflammation and promote systemic [Insulin Resistance] IR [10]. Furthermore, RBP4 may play a role in the pathogenesis of T2D by upregulating hepatic expression of the gluconeogenic enzyme phosphoenolpyruvate carboxykinase (PEPCK) and inhibiting insulin signaling in muscle [6]. Conversely, genetic deletion of RBP4 enhances insulin sensitivity [6]. Clinical studies in adults have demonstrated associations between RBP4 levels and obesity, IR, MS and T2D [5, 8, 9, 11], although not all studies agree [12,13,14].” […]
Abstract: “Leptin and adiponectin demonstrated the expected associations with metabolic disorders.” […] “Conclusions: Childhood RBP4 serves as a risk factor for subsequent development of [Metabolic Syndrome] MS and its components, independent of pediatric obesity. Incorporating childhood RBP4 into conventional cardiometabolic risk assessment models significantly improves the prediction of MS.” […]
Background: “In two longitudinal studies of overweight or obese youth, reductions in RBP4 were reported to accompany weight loss, improvement of triglyceride (TG) levels and [Insulin Resistance] IR [18, 19],” (Li, et al., 2018)
Psoriasis -
Higher RBP4 levels were associated with more severe symptoms. (Niu, et al., 2024) RBP4 levels were within ‘normal’ range and lower than those seen in patients with Coronary Artery Disease. (Ke, et al., 2022)
Severity of psoriasis is also associated with elevated RBP4: “RBP4 at baseline was higher in psoriasis patients than in healthy subjects [median (interquartile range): 13.39 (9.71–22.92) versus 9.59 (6.57–13.72) µg/mL] (P = 0.003).” (Niu, et al., 2024) Math: 1.339 (0.971-2.292 ml/dL) in the psoriasis patients vs 0.959 (0.657-1.372 ml/dL) in the control group. That suggests that a ‘normal’ range for RBP might be lower than the Labcorp ranges of “0 to 12 y - 1.2−4.6; 13 to 60 y - 1.6−6.1; 61 to 80 y - 1.8−7.3; >80 y - 1.6−6.1 mg/dL” (Labcorp/RBP) The control group people (with normally growing skin) had RBP values averaging 0.959 (0.657-1.372 ml/dL) - that would be considered lower than the normal range — and deficient in vitamin A potentially and a doctor might suggest a supplement.
Elevated retinoids cause skin to grow abnormally - the immune response that Retinoic Acid promotes is telling the body that there is a wound there, so grow a ~ scab, call for extra white blood cells to flood the area, instead of growing normal skin.
Alzheimer’s dementia - elevated seen in late stages, but low levels in CSF have been observed.
In Alzheimer’s dementia, elevated RBP4 has been observed in late stages, but very low levels in the cerebrospinal fluid have also been observed (lack of protein intake might be the dietary problem then). The research team did not find a difference in RBP4 levels between a small group with early AD (38 people) and a larger control group (113 people) “(preclinical AD: 20 men, 18 women; control: 45 men, 73 women)”. (Ishii, et al., 2019) However, the average for all of the participants, pre-clinical AD and control group, was at or above 3 mg/dL (see excerpt below), which would have put them more at risk of Major Adverse Cardiovascular Events (MACEs) in the study by Ke, et al., 2022. Below 3 mg/dL had less risk of MACEs. (Ke, et al., 2022) The control group without psoriasis had lower than normal levels averaging 0.959 (0.657-1.372 ml/dL). (Niu, et al., 2024)
“A few human studies have investigated the potential link between RBP4 and AD. A postmortem brain study with a limited number of subjects found that brain RBP4 levels were increased in AD subjects compared to non-AD control subjects [11]. In another small study, the cerebrospinal fluid (CSF) RBP4 levels were decreased in AD subjects compared to control subjects [12]. A more recent study found CSF RBP4 levels to decrease gradually from normal controls to mild cognitive impairment (MCI) and eventually be very low or absent in the more severe cases of AD [13].” […]
“Plasma RBP4 levels were similar between preclinical AD and control subjects in men (preclinical AD: 30.0 ± 7.4 µg/mL; control: 30.0 ± 8.7 µg/mL; p = 0.97) and women (preclinical AD 30.9 ± 7.9 µg/mL; control: 31.7 ± 8.5 µg/mL; p = 0.72).” (Ishii, et al., 2019)
Math - men, preclinical AD: 30 average, (22.6-37.4) µg/mL = 3.0 (2.26-3.74 mg/dL); control: 3.0 (2.13-3.87) and women (preclinical AD 3.09 (2.3-38.8) mg/dL; control: 3.17 (2.32-4.02). (Ishii, et al., 2019) All of those people would be in the more severe psoriasis group in the study by Niu, et al., 2024 and the ones with levels above 3 would be in the group at higher risk for a major cardiovascular event (if heart disease was present) in the study by Ke, et al., 2022. This suggests that other factors are involved in risks associated with RBP4 in addition to activated retinoids vs inactive retinol (vit. A). Lack of protein intake or zinc could explain there being very low levels of RBP4 in cerebrospinal fluid of patients with more severe Alzheimer’s. (Ishii, et al., 2019)
Personal experience - low protein had been a problem for years in the years prior to my mother developing Alzheimer’s dementia - histamine excess was a problem, but retinoids in her diet don’t seem to make her worse, while histamine trigger foods do.
Retinoid Toxicity is not very well researched except for cases involving retinoid medications - which are used for acne and anti-aging skin care in dermatology and cosmetic products, and for cancer treatment.
Most of the research on Retinoid Toxicity is from use of retinoid medications, and therefore, very high levels may be what is considered “Retinoid Toxicity”, while lower ‘high’ levels may also be causing damage - just less overt, acute damage.
Chronic degenerative conditions tend to be given a label and the symptoms are treated. The problem with the modern medical industry is that they wait way too long before calling health changes a condition worth treating. “Hypochondria” may be the unspoken diagnosis until something really clearly bad is seen - and then a diagnostic label and symptom treatment is likely. BUT it is within the earlier stages of chronic illness when damage can be reversible though. Waiting too long leads to more disability and discomfort and the increased likelihood of irreversible damage having occurred.
RBP in Chronic Kidney Disease - urinary levels is diagnostic, but not readily available to doctors for patient care. Radiation (aka EMF exposure too) can worsen risks.
Aside - pomegranate/peel has been shown to be helpful against kidney damage from toxin exposure - such as phenylhydrazine used to cause damage in an animal model of kidney disease. (Soliman, et al., 2023) Phenylhydrazine can be naturally occurring in a commercial mushroom, 500 mg per kilogram fresh weight of Agaricus bisporus mushrooms. It is a drug used to create hemolytic anemia in lab animals. (Anderson and Gry, 2004) Pomegranate/peel helps against Retinoid Toxicity and histamine excess by reducing inflammation and inhibiting degranulation of mast cells. It is protective against kidney, liver, brain, or heart disease and against cancer, or various infections.
See Version 1. Pomegranate, TRP channels, nociceptive pain and endocannabinoids (transcendingsquare.com)
RBP has regulatory functions over where the activated retinoids are taken.
RBPs also have regulatory function over retinoid metabolism and disposition - where it is being taken - the Uber driver knows the way…. hopefully. RBP levels also tend to be elevated in chronic kidney disease (CKD). (Uremic Toxicity, 2023, viewable at Retinol Binding Protein)
“Retinol binding proteins (RBPs) are not only transporters of retinoids but also function as regulators of retinoid metabolism and disposition. They have extensively been studied in CKD and were repeatedly shown to be elevated, although levels do not correlate as well with GFR than cystatin C or β2-microglobulin. Uremic Toxicity, Raymond Vanholder MD, PhD, Griet Glorieux PhD, in Handbook of Dialysis Therapy (Sixth Edition), 2023 viewable at (Retinol Binding Protein)
RBP functions can be contradictory. It has been shown to cause cell death, apoptosis and increase inflammation in human endothelial cells, but also has been observed to inhibit apoptosis when taken from a patient with acute kidney failure. Other studies link RBP levels with metabolic syndrome (read RBP as “RBP and the activated retinoids it carries”). (Uremic Toxicity, 2023, viewable at Retinol Binding Protein)
“Contradictory biological functions have been attributed to this molecule. On one hand, RBP has been shown to induce apoptosis and inflammatory reaction in human endothelial cells. On the other hand, RBP isolated from acute kidney failure patients inhibited polymorphonuclear cell function and apoptosis. Quite a number of observational studies in specific populations not necessarily suffering from CKD link RBP to CV disease, carotid intima thickness, metabolic syndrome, and obesity. Only a few observational studies assessed links of RBP with outcomes in specific populations suffering from CKD. In one study, serum levels were associated with components of metabolic syndrome. In a population of type 2 diabetics with and without CKD, serum RBP was, however, not related to carotid intima thickness.” Uremic Toxicity, 2023, viewable at (Retinol Binding Protein)
Worse kidney function was associated with higher levels of urinary RBP, and greater Cardiovascular risk factors. Levels of RBP in the urine was increased with more severe levels of Radiation damage to the kidneys (Uremic Toxicity, 2023, viewable at Retinol Binding Protein) — which EMF exposure would be similar too. Reading “Elevated serum RBP” as “Elevated Retinoic Acid or other Retinoids” makes it a lot more obvious that chronic kidney disease or Metabolic syndrome or cardiac disease might all really be undiagnosed Retinoid Toxicity and/or zinc and taurine deficiency.
On the other hand, urinary RBP was inversely related to kidney function and CV risk factors and was also increased in proportion with the degree of kidney radiation damage in mice.”
*Uremic Toxicity, Raymond Vanholder MD, PhD, Griet Glorieux PhD, in Handbook of Dialysis Therapy (Sixth Edition), 2023 viewable at (Retinol Binding Protein)
Food sources of vitamin A and carotenoids are plentiful in the modern diet.
Tomato products can be in every meal very easily: pizza, pasta, salsa, salads, ketchup, barbecue sauce, curries, canned soup, or liver and onions, or carrot and kale smoothies, many common ‘healthy’ foods could be adding to Metabolic Syndrome, heart or kidney disease…. or adding to skin rashes and maybe hair loss. Many supplements contain vitamin A and carotenoids or may contain it as part of the natural ‘carrot-derived’ food coloring. Snack crackers and other foods may also have carrot or tomato added for color and flavor.
The popularity of the Carnivore diet has made Retinoid Toxicity from liver intake more common. Kale and carrot smoothies have also sickened people from excess carotenoids. It is believed to be not dangerous to overeat carrots - the skin turns orange is all…. but that may not be true. Vitamin A collects in the liver and following a restricted diet can take a long time to deplete the stored fat-soluble carotenoids.
Unfortunately, the RBP urinary lab test isn’t in use yet for general patient care yet. Lab tests showing low or normal RBP in the blood may also not reflect a liver that is overfull of retinoids that aren’t being released due to a lack of protein or zinc or taurine. The serum level of RBP is a start though — check and see if it is elevated as that has been linked to insulin resistance, Metabolic Syndrome, and cardiovascular disease. Retinoid Toxicity is also linked to liver, kidney, and brain damage over time. See: Table 1. Symptoms of Histamine and Retinoid Toxicity (Dropbox)
Is vitamin A good for us or bad for us? Or is it that lack of zinc and protein is bad for us? And/or lack of sunshine?
Plenty of sunshine helps reduce vitamin A levels by using it up and that also helps keep it in balance with vitamin D because more vitamin D would be created. Eating things seasonally may matter - in the summer we have lots of carotenoid rich greens to eat, and sunshine to help us use the carotenoids as antioxidants.
RBP or IRBP?
It may also be called Retinoid Binding Protein, and Interphotoreceptor retinoid-binding protein (IRBP) (Liou, et al., 1991) is also known as RBP3. (Zeng, et al., 2020) IRBP is involved in light reception within the retina.
Just to keep us all on our toes… some more alphabet soup.
Activated retinoids are even controlled on transport proteins within cells - called cellular retinol-binding proteins (CRBPs).
“Plasma retinol-binding protein ... cellular retinol-binding proteins (CRBPs) and cellular retinoic acid-binding proteins (CRABPs) carry retinoids within cells [2]. RBP, ERABP, CRBPs (CRBP I, II, III, and IV) and CRABPs (CRABP I and CRABP II) belong to the lipocalins” (Zhang, et al., 2012) *Lipocalins are a larger group of transport proteins which tend to be able to bind iron in addition to some other molecule or mineral.


More on retinoic acid and synaptic plasticity

*Parvalbumin is in an example to look at closer - (Zhong, et al., 2018)
Protons, from acidosis in the brain in general or a localized mechanism, can act as a signaling neurotransmitter in the amygdala. (Du, et al., 2014)
RBP and prealbumin are teammates during the process of transporting activated vitamin A (Retinoic acid / retinoids) to cells. After the cell takes in the retinoid the complex separates into RBP and prealbumin again. (Rask, et al., 1980)
“Vitamin A is transported from its storage site in the liver to the epithelial tissues by a carrier protein, the Retinol-binding protein (RBP). In plasma RBP forms a complex with thyroxine-binding prealbumin. The plasma concentration of RBP is regulated by the vitamin A status so that in vitamin A deficiency RBP molecules are not secreted from the liver. RBP molecules interact with a cell membrane receptor, probably a protein component present on epithelial cells. Vitamin A is thereby delivered to the cells. The uptake of vitamin A by the cells causes a reduction of the affinity of RBP for prealbumin. The RBP molecules which no longer are able to interact efficiently with prealbumin are excreted through the kidney glomerulus and degraded.” (Rask, et al., 1980)
[*In healthy kidneys the RBP may be conserved for reuse rather than being excreted.]
My having retinoid toxicity and albumin sensitivity issues may be related. I don’t know though. I have another post started that gets into how albumin may interact with hair follicles.
Disclaimer: This information is being provided for educational purposes within the guidelines of Fair Use and is not intended to provide individual health care guidance.
Reference List
(Cassandri, et al., 2017) Cassandri, M., Smirnov, A., Novelli, F. et al. Zinc-finger proteins in health and disease. Cell Death Discov. 3, 17071 (2017). https://doi.org/10.1038/cddiscovery.2017.71 https://www.nature.com/articles/cddiscovery201771.
(Du, et al., 2014) Du J, Reznikov LR, Price MP, Zha XM, Lu Y, Moninger TO, Wemmie JA, Welsh MJ. Protons are a neurotransmitter that regulates synaptic plasticity in the lateral amygdala. Proc Natl Acad Sci U S A. 2014 Jun 17;111(24):8961-6. doi: 10.1073/pnas.1407018111. Epub 2014 Jun 2. PMID: 24889629; PMCID: PMC4066526. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4066526/
(Fahim, et al., 2022) Fahim SM, Gazi MA, Alam MA, Hasan MM, Das S, Mahfuz M, Ahmed T. Association between Circulating Retinol Binding Protein 4, Body Mass Index, and Biomarkers of Environmental Enteric Dysfunction among Slum-Dwelling Lean Adults in Bangladesh. Am J Trop Med Hyg. 2022 Oct 10;107(6):1315-1322. doi: 10.4269/ajtmh.21-0322. PMID: 36216318; PMCID: PMC9768260. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9768260/.
(Ishii, et al., 2019) Ishii M, Kamel H, Iadecola C. Retinol Binding Protein 4 Levels Are Not Altered in Preclinical Alzheimer's Disease and Not Associated with Cognitive Decline or Incident Dementia. J Alzheimers Dis. 2019;67(1):257-263. doi: 10.3233/JAD-180682. PMID: 30562901; PMCID: PMC6385158. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6385158/.
(Kanai, et al., 1968) Kanai M, Raz A, Goodman DS. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest. 1968 Sep;47(9):2025-44. doi: 10.1172/JCI105889. PMID: 5675424; PMCID: PMC297364. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC297364/.
(Ke, et al., 2022) Ke, Q., Xin, Y., Cheng, X., Yijia, F., Moshuang, M., Association Between Circulating Retinol-Binding Protein 4 and Adverse Cardiovascular Events in Stable Coronary Artery Disease, Frontiers in Cardiovascular Medicine, Vol 9, 2022, DOI=10.3389/fcvm.2022.829347, ISSN=2297-055X, https://www.frontiersin.org/journals/cardiovascular-medicine/articles/10.3389/fcvm.2022.829347.
(Kedishvili, 2016) Kedishvili NY. Retinoic Acid Synthesis and Degradation. Subcell Biochem. 2016;81:127-161. doi: 10.1007/978-94-024-0945-1_5. PMID: 27830503; PMCID: PMC5551983. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5551983/
(Kim, et al., 2022) Kim HJ, Zhao J, Sparrow JR. Vitamin A aldehyde-taurine adducts function in photoreceptor cells. Redox Biol. 2022 Aug;54:102386. doi: 10.1016/j.redox.2022.102386. Epub 2022 Jul 3. PMID: 35809434; PMCID: PMC9287728. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9287728/
(Li, et al., 2018) Li, G., Esangbedo, I.C., Xu, L. et al. Childhood retinol-binding protein 4 (RBP4) levels predicting the 10-year risk of insulin resistance and metabolic syndrome: the BCAMS study. Cardiovasc Diabetol 17, 69 (2018). https://doi.org/10.1186/s12933-018-0707-y https://cardiab.biomedcentral.com/articles/10.1186/s12933-018-0707-y.
(Liou, et al., 1991) Liou GI, Geng L, Baehr W. Interphotoreceptor retinoid-binding protein: biochemistry and molecular biology. Prog Clin Biol Res. 1991;362:115-37. PMID: 2003123. https://pubmed.ncbi.nlm.nih.gov/2003123/.
(Morris and Levenson, 2013) Morris DR, Levenson CW. Zinc regulation of transcriptional activity during retinoic acid-induced neuronal differentiation. J Nutr Biochem. 2013 Nov;24(11):1940-4. doi: 10.1016/j.jnutbio.2013.06.002. Epub 2013 Sep 9. PMID: 24029070; PMCID: PMC3832953. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3832953/.
(Muenzner, et al., 2013) Muenzner M, Tuvia N, Deutschmann C, Witte N, Tolkachov A, Valai A, Henze A, Sander LE, Raila J, Schupp M. Retinol-binding protein 4 and its membrane receptor STRA6 control adipogenesis by regulating cellular retinoid homeostasis and retinoic acid receptor α activity. Mol Cell Biol. 2013 Oct;33(20):4068-82. doi: 10.1128/MCB.00221-13. Epub 2013 Aug 19. PMID: 23959802; PMCID: PMC3811689. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3811689/.
(Niu, et al., 2024) Niu, R., Li, Z., Jiang, W. et al. Pre-treatment plasma retinol binding protein 4 level and its change after treatments predict systemic treatment response in psoriasis patients. BMC Immunol 25, 55 (2024). https://doi.org/10.1186/s12865-024-00647-7 https://bmcimmunol.biomedcentral.com/articles/10.1186/s12865-024-00647-7.
(Qi, et al., 2007) Qi Q, Yu Z, Ye X, Zhao F, Huang P, Hu FB, Franco OH, Wang J, Li H, Liu Y, Lin X. Elevated retinol-binding protein 4 levels are associated with metabolic syndrome in Chinese people. J Clin Endocrinol Metab. 2007 Dec;92(12):4827-34. doi: 10.1210/jc.2007-1219. Epub 2007 Sep 18. PMID: 17878249. https://academic.oup.com/jcem/article/92/12/4827/2597683?login=false.
(Quadro, et al., 2003) Quadro L, Hamberger L, Colantuoni V, Gottesman ME, Blaner WS. Understanding the physiological role of retinol-binding protein in vitamin A metabolism using transgenic and knockout mouse models. Mol Aspects Med. 2003 Dec;24(6):421-30. doi: 10.1016/s0098-2997(03)00038-4. Erratum in: Mol Aspects Med. 2004 Jun;25(3):361. PMID: 14585313. https://pubmed.ncbi.nlm.nih.gov/14585313/#:~:text=The%20single%20known%20function%20of,hepatic%20stores%20to%20target%20tissues.
(Rask, et al., 1980) Rask L, Anundi H, Böhme J, Eriksson U, Fredriksson A, Nilsson SF, Ronne H, Vahlquist A, Peterson PA. The retinol-binding protein. Scand J Clin Lab Invest Suppl. 1980;154:45-61. PMID: 7010520.
Retinol Binding Protein - an overview, ScienceDirect.com, https://www.sciencedirect.com/topics/medicine-and-dentistry/retinol-binding-protein#:~:text=12%20RBP-,RBP%2C%20acronym%20of%20Retinol%20Binding%20Protein%20is%20a%20protein%20synthesized,sensitive%20marker%20of%20tubular%20injury.
(Smith, 1980) Smith JC Jr. The vitamin A-zinc connection: a review. Ann N Y Acad Sci. 1980;355:62-75. doi: 10.1111/j.1749-6632.1980.tb21328.x. PMID: 6786155. https://nyaspubs.onlinelibrary.wiley.com/doi/abs/10.1111/j.1749-6632.1980.tb21328.x?sid=nlm%3Apubmed.
Side topic: (Soliman, et al., 2023) Pomegranate/peel protective against toxin damage in the kidneys. Soliman NA, Mansour SW, Ammar MA, Hassan NA, Mohamed RHA. Possible role of pomegranate fruit in reversing renal damage in rats exposed to Phenylhydrazine. Open Vet J. 2023 Oct;13(10):1268-1276. doi: 10.5455/OVJ.2023.v13.i10.5. Epub 2023 Oct 31. PMID: 38027401; PMCID: PMC10658015. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10658015/
*related to Soliman, Phenylhydrazine can be naturally occurring in a commercial mushroom, 500 mg per kilogram fresh weight of Agaricus bisporus mushrooms. It is used to create hemolytic anemia in lab animals. (Anderson and Gry, 2004) Andersson, H. C., Gry, Jørn, Phenylhydrazines in the cultivated mushroom (Agaricus bisporus), Technical University of Denmark, 2004, ISSN:09086692 https://orbit.dtu.dk/en/publications/phenylhydrazines-in-the-cultivated-mushroom-agaricus-bisporus
(Steinhoff, et al., 2022) Steinhoff JS, Lass A, Schupp M. Retinoid Homeostasis and Beyond: How Retinol Binding Protein 4 Contributes to Health and Disease. Nutrients. 2022 Mar 15;14(6):1236. doi: 10.3390/nu14061236. PMID: 35334893; PMCID: PMC8951293. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8951293/
(Sun, et al., 2013) Sun Q, Kiernan UA, Shi L, Phillips DA, Kahn BB, Hu FB, Manson JE, Albert CM, Rexrode KM. Plasma retinol-binding protein 4 (RBP4) levels and risk of coronary heart disease: a prospective analysis among women in the nurses' health study. Circulation. 2013 May 14;127(19):1938-47. doi: 10.1161/CIRCULATIONAHA.113.002073. Epub 2013 Apr 12. PMID: 23584360; PMCID: PMC3741657. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3741657/.
(Vilhais-Neto and Pourquié, 2008) Gonçalo C. Vilhais-Neto and Olivier Pourquié, Retinoic Acid, Current Biology, Vol 18, Issue 7, 8 April 2008, Page 550–552 DOI:https://doi.org/10.1016/j.cub.2008.03.032 https://www.dropbox.com/scl/fi/zdzhhxa3tjpiuv8id7occ/PIIS0960982207024396.pdf?rlkey=6zxak2gvzgap1d10wrttc7670&dl=0
(Zabetian-Targhi, et al., 2015) Zabetian-Targhi F, Mahmoudi MJ, Rezaei N, Mahmoudi M. Retinol binding protein 4 in relation to diet, inflammation, immunity, and cardiovascular diseases. Adv Nutr. 2015 Nov 13;6(6):748-62. doi: 10.3945/an.115.008292. PMID: 26567199; PMCID: PMC4642414. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4642414/.
(Zeng, et al., 2020) Zeng, S., Zhang, T., Madigan, M.C., Fernando, N., Aggio-Bruce, R., Zhou, F., Pierce, M., Chen, Y., et al.,, Interphotoreceptor Retinoid-Binding Protein (IRBP) in Retinal Health and Disease, Frontiers in Cellular Neuroscience, 14, 2020, DOI=10.3389/fncel.2020.577935 https://www.frontiersin.org/journals/cellular-neuroscience/articles/10.3389/fncel.2020.577935/full.
(Zhang, et al., 2012) Zhang YR, Zhao YQ, Huang JF. Retinoid-binding proteins: similar protein architectures bind similar ligands via completely different ways. PLoS One. 2012;7(5):e36772. doi: 10.1371/journal.pone.0036772. Epub 2012 May 4. PMID: 22574224; PMCID: PMC3344936. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3344936/.
(Zhong, et al., 2018) Zhong LR, Chen X, Park E, Südhof TC, Chen L. Retinoic Acid Receptor RARα-Dependent Synaptic Signaling Mediates Homeostatic Synaptic Plasticity at the Inhibitory Synapses of Mouse Visual Cortex. J Neurosci. 2018 Dec 5;38(49):10454-10466. doi: 10.1523/JNEUROSCI.1133-18.2018. Epub 2018 Oct 24. PMID: 30355624; PMCID: PMC6284108. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6284108/
Zinc Finger Proteins, TRANSCRIPTION FACTORS | Overview, I.M. Adcock, ... G. Caramori, in Encyclopedia of Respiratory Medicine, 2006; via (ScienceDirect/Zinc finger proteins)
https://x.com/NutriDetect/status/1604520929286975489?t=92VC_olwS4RC6O3WbiN11g&s=19.
https://x.com/UnrollHelper/status/1604570089826689027?t=jyQqRe8IBnZ64ZbgPZ_CkA&s=19.
https://nutritiondetective.com/pages/madness-of-modern-nutrition-course.







Extra supplementation during infection helps with
measles and respiratory syncytial virus (RSV)enteric infections,covid,norovirus, Mycoplasma Pneumoniae(TB),cytomegalovirus (and cause of its epithelial cell barrier enhancement,parasites ,Giardia, Borrelia)
Understanding the role of vitamin A and its precursors in the immune system
https://www.sciencedirect.com/science/article/abs/pii/S0985056221002156
Effects of vitamin A supplementation on intestinal barrier function, growth, total parasitic, and specific Giardia spp infections in Brazilian children: a prospective randomized, double-blind, placebo-controlled trial
Conclusions: These data showed that total parasitic infection and Giardia spp infections were significantly lower in the vitamin A treatment group when compared with the placebo group, suggesting that vitamin A improves the host's defenses against Giardia infections.