CYM Co. adrenochrome sales data leak; 1967 book on adrenochrome's discovery, coauthor Dr. Hoffer - the original niacin guy. Adrenochrome use -> Personality changes & schizophrenia risk.
Diet & lifestyle tips for reducing risk of excess adrenochrome production or accumulation, as it can cause symptoms like schizophrenia, which seems to be multi-factorial, not a single-cause condition.
Topic one -Big data leak about a company offering adrenochrome for sale that is sourced from human blood. There is ~31 pages, including a Table of children’s names and ages, from CYM Corporation, with prices for vials of adrenochrome for sale. It seems too comprehensive to be a LARP, a faked ‘hack’. Click for video: Adrenochrome Production Exposed, (rumble.com).
Topic two - the chemistry of adrenochrome metabolism and how it relates to schizophrenia - and why the use of high dose niacin often helps patients with schizophrenia. Excerpts and a summary of a 1967 book chapter coauthored by Dr. Abram Hoffer. He is a research scientist who showed that high dose niacin can successfully treat schizophrenia. Two new graphics summarize the dietary tips.
Chapter III. Adrenochrome and Some of Its Derivatives, The Hallucinogens, by A. Hoffer and H. Osmond, 1967, Academic Press, 181 pages, (pdf in my Dropbox)
This post is also available as a document with a clickable Table of Contents: Adrenochrome & Schizophrenia, (sync/pdf).
Links mentioned in the video, are copied below. One is a file dump, copy in my Sync file (Sync.com, pdf).
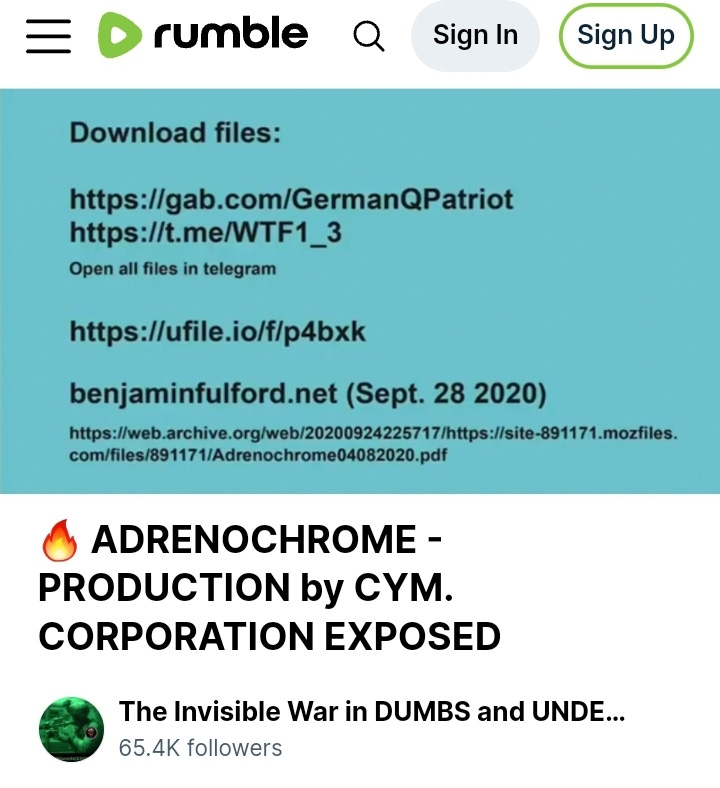
Click for video: (rumble.com)
https://gab.com/GermanQPatriot ← this is an account on Gab.com, open there to follow or view the account’s posts.
Https://t.me/WTF1_3 ← this is a Telegram channel, open in Telegram so you can join or view the channel posts.
“open all files in telegram”
https:/ufile.io/f/p4bxk ← page not found
benjaminfulford.net (Sept. 28 2020), ← a blog site. ‘Satanic Dutch Royal Family executed? – Pentagon sources’, By Benjamin Fulford, September 28, 2020, (benjaminfulford.net/satanic-dutch-royal-family-executed-pentagon-sources/)
The File dump, 30 pages, CYM Corp., image of an email below. https://web.archive.org/web/20200924225717/https://site-891171.mozfiles.com/files/891171/Adrenochrome04082020.pdf . . . CYM files, of the 30 pages, many are of a dataset, a Table of children’s names, their blood types and their ages – under ten y/o frequently. Copy in my (Sync.com, pdf)

Question - If elites are using adrenochrome rich blood (of terrorized children) for anti-aging or other purposes, then it likely is also causing a lack of empathy, poor judgement, and an increased error rate. Are we seeing that?
Thanks go to fellow Substacker,
, Substack: Sense of Awareness, for sharing this chapter about the chemistry of adrenochrome and its role in the mental and physical health of patients with schizophrenia, (on Telegram).Chapter III. Adrenochrome and Some of Its Derivatives, The Hallucinogens, by A. Hoffer and H. Osmond, 1967, Academic Press, 181 pages, (pdf in my Dropbox)
Two main points -
Schizophrenia patients are still being given electroshock ‘treatment’ instead of high dose niacin - proven beneficial by Dr. Hoffer and used clinically since 1971.
Pedo-people and adrenochrome users seem to be in control of much of society and they are likely dangerous monsters who do not believe that they are dangerous monsters, because of the effects of adrenochrome on their psyche.
Subpoints - the chemistry of adrenochrome reveals what dietary changes might help prevent schizophrenia like symptoms and the later part of this post focuses on how people might improve their health or prevent problems from stress and fear.
A previous series on risk factors for schizophrenia ended up with an extensive list of nutrients or dietary issues that might be causally linked.
See for a later post in the lengthy series: Going out on a limb - #25 - excess Glyphosate. #26 - Microbiome health. #27 - low Tryptophan, (denutrients.substack)
*One additional modern cause of schizophrenia-like symptoms, not discussed in the book, are the very high dose THC products available now in medical and recreational legal states. Without some CBD to balance the euphoria inducing THC, the person can become disoriented in thinking similarly to schizophrenia. What is needed is CBD to balance, and less THC. Genetic differences in cannabinoid metabolism are seen with schizophrenia - the issue being a lack of the CBD equivalent, 2-AG, and an excess of the THC equivalent, anandamide. See this post: Clinical Endocannabinoid Deficiency, (CED), and phospholipids. (denutrients.substack).
These info-graphics are summaries of information included later in this post. Anyone under severe stress and anxiety and who has depleted antioxidants and methylation and sulfation function may be at risk for excess production and accumulation of adrenochrome.

*I could use a Part 3 for phosphorus and calcium balance, and the need for good vitamin D and K2 metabolism, and Vit D Binding transport protein (also called GcMAF). And copper/zinc balance can also be an issue in schizophrenia risk. Estrogen may be protective, increasing risk for post-menopausal women to have a new onset of mental illness symptoms.

The users of adrenochrome may develop reckless behavior and apathetic disregard for consequences to others or themselves. An academic text chapter on adrenochrome shares these behavior risks from their own trials and experiences observing volunteers - adrenochrome was a new drug being isolated and tested, as a synthesized metabolite of adrenalin.
The researchers Hoffer and Osmund, and their teams, were on the cutting edge of science - discovering the properties and isolation methods of new chemicals.
*Name dropping tangent - Hoffer and Osmund got some of the adrenolutin from Pfizer. It was unstable and hard to obtain, not a standard lab chemical.
The researchers tried the hallucinogens for themselves - first mescaline and LSD and then the adrenolutin and then adrenochrome. They took their own notes about self use, and had observer scientists also take notes about apparent changes related to the newly trialed drugs. Comparing the sets of notes revealed consistently that adrenochrome or adrenolutin caused significant behavior changes in a person BUT the person often didn’t believe it - and might even get mad about that feeling. They would think they had been given a placebo or a dud. They often thought that no change had occurred, or maybe a little bit of a depressant effect, even though unusual irritability or other changes were obvious to their secretaries/staff or the observer scientists who were familiar with the person.
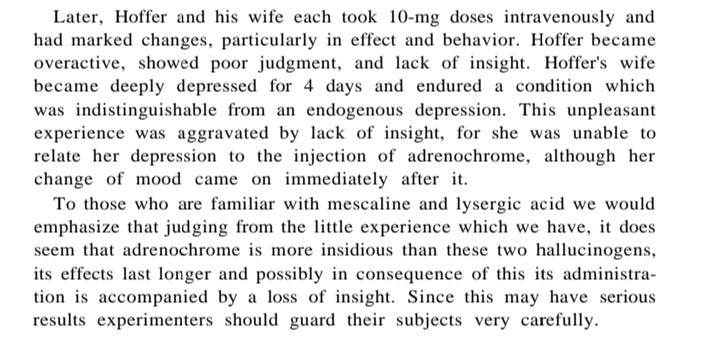
Another example - changes in perception but along with a feeling that nothing had happened - disappointed volunteer, but the observer saw distorted thinking, irritability and a lack of concern for others - but no awareness of those changes. Depression in the volunteer was also observed.
Dangerous people who don't realize they are dangerous, are likely to be more dangerous than people who recognize they are a risk to themselves or others.
Reckless behavior - and apathy or disregard for it, is a Risk of having elevated adrenochrome
- and personality changes may remain sometimes after use - observations of the scientists testing it on themselves and other volunteers.
The chapter Adrenochrome and Some of Its Derivatives, by Hoffer and Osmond, 1967, includes detailed accounts by the researchers who tried the newly discovered adrenochrome -- synthesized from adrenalin or adrenolutin rather than being purified from blood. The first-hand experiences written after the trial and observers accounts are included.
We may all need to take note of these side effects, if there are people in positions of power who are regular users of adrenochrome - apathetic disregard for consequences of their actions… is not an appealing trait in a government or civil leader.
»> The biggest take home point is that most of the people trying the 1967 era adrenochrome did not self-recognize how changed their behavior was while under the influence of adrenochrome. They would get angry and say that nothing had happened — the new drug was a dud even. “You gave me a dud!” — uncharacteristically angrily. Or paranoid and suspicious, but not seeing that it was a “themself” issue. The personality differences were subtle to outside observers. The users didn’t look “drugged” - it would be the long-known secretary or friend or coworker who would know that angrily snapping or rude behavior was out of norm.
Loss of good judgement, paranoia, irritability were all frequent symptoms along with changes in depth perception and other visual hallucinatory effects.
Reckless behavior, dumb mistakes: That it was no longer safe for themselves to drive was perceived by most who tried it, but the researchers suggest not leaving people alone when using adrenochrome for research or therapy purposes as some car accidents happened during trials of the new drug. Behavior was likened to that of High pressure oxygen situations, like Deep-sea divers who come up too fast. They can become very reckless in their behavior, taking off their air-tank while under water, or trying to leave the decompression chamber too early. (Brave AI summary).
This was early days in the discoveries - a brand-new chemical being isolated. Throughout the chapter the authors make it clear that there was controversy at the time - discovery of adrenochrome/adrenalin metabolism was being questioned or was disbelieved. (Hoffer and Osmond, 1967)
In a person with schizophrenia, increases in adrenaline get turned into adrenochrome instead of safer aldehydes or sulfates.
Adrenaline and adrenochrome are so reactive that it is stored in red blood cells because RBCs are so different than other cells - they have no cell nucleus with DNA that the reactive adrenaline chemistry would endanger.
*Someone with poor sulfur metabolism from genetics, or with an excess of sulfur chemicals from diet, supplements or Epsom salt baths, might be increasing adrenochrome production from metanephrine in Fig. 8 below, instead of it being converted into sulfates. that is a guess though, rather than from the text.

Figure 8. A scheme relating adrenaline destroying enzymes to psychotomimetic activity. A = monoamine oxidase, B = adrenaline oxidase, C = sulfoesterase.
Psychotomimetic: monoamine oxidase inhibitors [a huge class of anti-depressant medications] and adrenaline oxidase activators.
Antipsychotic: monoamine oxidase activators and adrenaline oxidase inhibitors. (Hoffer and Osmond, 1967)
Adrenaline oxidase (*oxidizes adrenalin) is the enzyme to inhibit (B) in Figure 8, and monoamine oxidase (A) is the one to promote, to help someone with schizophrenia. Details on phytonutrient MAO activators would be another topic. MAO inhibitors are a large class of antidepressant medications. Search results for adrenaline oxidase direct me to MAO information, so the chemical term may be out of date - I don’t know enough about the chemistry and didn’t dig further at this time.
Brave AI - Based on the provided search results, the following factors can increase the activity of monoamine oxidase (MAO):
Calcium (Ca2+): Calcium has been shown to selectively increase the activity of MAO-A, a mitochondria-bound enzyme that generates peroxyradicals as a natural by-product of the deamination of neurotransmitters such as serotonin. This calcium-sensitive regulation of MAO-A contributes to the production of peroxyradicals in hippocampal cultures, which may be implicated in Alzheimer’s disease-related pathology (AD) -
*Excessive activity is linked to neurodegenerative conditions including AD. (Cao, et al., 2007; Yeung, et al., 2019) This means having adequate vitamin D and vitamin D binding protein for transport is necessary, along with adequate vitamin K2 to keep calcium, phosphorus, and magnesium in the bone matrix, instead of having excess calcium in the soft tissues or a phosphorus imbalance in the blood - seen with overacidity which increases inflammation and oxidative stress chemicals.
**Lack of monamineoxidase enzyme A and B is also associated with neurocognitive conditions: ADHD, Alzheimer’s dementia, and Autism Spectrum Disorder. (Bortolato and Shih, 2011)
Estrogens: Estrogens, particularly in postmenopausal women [observed with estrogen replacement therapy], have been found to play a protective role in the progression of Alzheimer’s disease by contributing to *down-regulation of MAO, but up-regulation has also been observed. (Chakravorty and Halbreich, 1997; Holschneider, et al., 1998)
*Resveratrol or soy genistein might be supportive then, as they are phytonutrient estrogen receptor agonists.
When sulfation pathways are slow or dysfunctional, excessive ammonia can collect, (Brave AI summary/sulfation & ammonia), which inhibits the MAO enzymes, which otherwise would help prevent an excess of oxidative chemicals from forming. *Decreased methylation cycle function can negatively impact sulfation pathways too.
“MAO function is highly critical for the regulation the intracellular redox state in neurons and other cells; indeed, one of the byproducts of MAO-mediated reaction, hydrogen peroxide, is a potent oxidizer which can trigger the formation of superoxide radicals and other reactive oxygen species, which can in turn induce mitochondrial and cytoplasmic damage. Under physiological conditions, the overall redox potential is kept in equilibrium by antioxidant enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase; nevertheless, high concentrations of ammonia (the other by-product of the reaction) have been shown to decrease the activity of these enzymes and lead to the formation of superoxide radicals (Kosenko et al., 1997).” (Bortolato and Shih, 2011)
I have a gene difference in my MAO enzyme and I have slow sulfate metabolism - causing a tendency to accumulate excess of the toxic sulfides/sulfites used as preservatives on dried fruit and in other things like processed meats. Also reducing high sulfur foods like garlic, turnips and cruciferous vegetables, can help the body to be able to keep up with sulfate metabolism. It naturally tends to be a little slow.
The biochemistry and gene alleles affecting the methylation and sulfation cycles get confusing. Sulfation problems can be due to a lack of methylation function too. Methylated adrenaline is not as toxic - the methyl transferase path is straight downwards in Figure 8 converting it into metanephrine. Sulfation would then deactivate the metanephrine instead of allowing it to be converted into adrenochrome. (Hoffer and Osmond, 1967)
Modern day Dr. Shiva Ayyadurai looked at the chemistry from the perspective of what is alleged to be done to children during harvesting of blood for adrenochrome - oxidation is needed. First the children are stressed. Fear is induced and adrenalin is created to run away, but then the children are tortured. A fear of death levels of anxiety needs to be induced for the adrenalin to be converted into adrenochrome. Click through for a brief video: (x.com/HamHam361791). Oxidation of adrenalin to adrenochrome, the B pathway in Figure 8 above, is being described. A lack of antioxidants would be an added risk factor - glutathione and ascorbic acid deficiency are mentioned in Table 27 below. (Hoffer and Osmond, 1967)
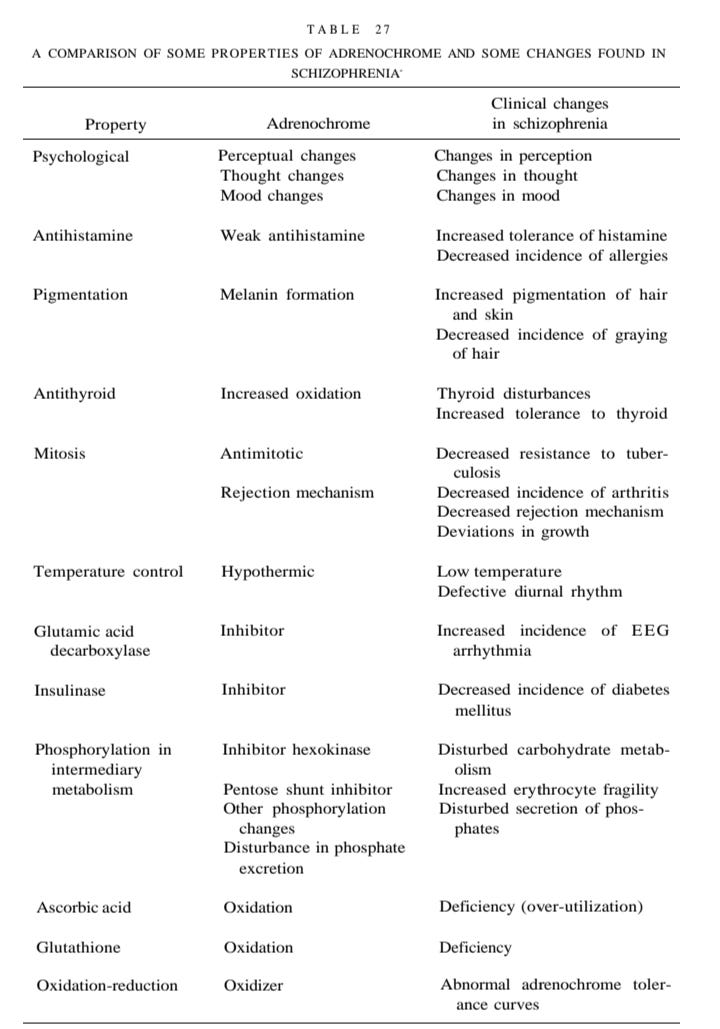
»> Nicotinic acid is the flush type of niacin. It is considered the “antidote” to adrenochrome and adrenolutin in the Table 27. A Comparison of Some Properties of Adrenochrome and Some Changes Found in Schizophrenia, below. (Hoffer and Osmond, 1967) A researcher who had a fairly bad experience and who needed to work clinically that night was given a high dose of niacin to help offset the adrenochrome trial. It worked. He regained normal thinking.
Effects on personality sometimes lingered a few days after the adrenochrome or adrenolutin trials. People's reactions would vary a fair amount between minimal effect and kind of bad effects.

This adrenochrome info was based on info already published in an older post on adrenochrome, this now edited/reformatted, and includes part 2 post. And part 3 was primarily a combination of the first two. This post covers all of the adrenochrome sections in those posts in more detail here.
Chapter III. Adrenochrome and Some of Its Derivatives, 1967, (pdf in my Dropbox) is a 181 page chapter on adrenochrome in a book called The Hallucinogens.
This book chapter is about very early research on adrenochrome, a synthesized version, in a lab. It answers the question: Why do schizophrenics usually benefit from high dose niacin?
»> Because people with schizophrenia might lack an enzyme that converts another chemical into nicotinic acid - which would reduce a need for a dietary source of niacin in a person with typical genetics.
More on schizophrenia and niacin: (Oxenkrug and Forester, 2024)]
One of the authors, Abram Hoffer, went on to clinically treat schizophrenic patients successfully with high dose niacin.
Schizophrenia is a focus of the book chapter on adrenochrome because use of the synthesized adrenochrome (unknown mystery chemical at earlier stages of research) caused similar symptoms, (and Hoffer was already involved in niacin research). So, the scientists looked closer at what is happening in patients with schizophrenia and they found there seems to be an abnormal ability to process adrenalin. Instead of normal breakdown pathways being used for adrenalin, excess adrenochrome is made from it. And then adrenochrome might accumulate in susceptible people, due to lack of nutrients to break it down properly. Excess of adrenochrome results in schizophrenia-like thinking. Reckless behavior and apathy was also observed when testing the synthesized adrenochrome.
Adrenochrome is the oxidized form of adrenalin, so lack of antioxidants would increase risk of an excess of oxidative stress chemicals ready to promote the conversion. Lack of our endogenously created glutathione, or its precursor amino acids cysteine and glycine, are risk factors for schizophrenia ~ aka excess adrenochrome production and reduced breakdown.
The gist seems to be that people with schizophrenia are not clearing adrenalin/adrenochrome adequately and accumulation of adrenochrome causes hallucinations and disordered thinking.
Adrenochrome excess can also lead to brown hair, may reduce aging, and can help preserve insulin supply, reducing risk for diabetes. Negative risks besides the mental symptoms include hypothyroidism, cold sensitivity, and histamine excess is common which can add to mental disorientation.
A visible characteristic of schizophrenia is brown hair: alternate breakdown of adrenochrome causes a brown colored version of melanin to form (rather than black which forms from dopachrome) and people with schizophrenia tend to get brown hair earlier as children (rather than stay blonde or have black hair) and to not go gray, white-haired, as soon as an older adult.
*Worsening cognitive health and Alzheimer’s dementia can be long-term risks of schizophrenia. (There is a previous post series on this topic - schizophrenia risk factors and their coinciding with Alz. risk factors - mitochondrial dysfunction and lack of mitochondrial support nutrients is an overlapping feature.)
*Personal experience, elevated histamine can cause very disoriented thinking too. Elevated histamine also is seen in schizophrenics, Retinoid Toxicity may be a factor, protein, zinc and taurine deficiency can be involved, mentioned later.
Why isn’t high dose niacin being used for schizophrenic patients? - is the link to adrenochrome chemistry causing it to be suppressed info?
High dose niacin is still only an alternative therapy for schizophrenia even though it has been in clinical use for the condition since the 1970s by Canadian Dr. Abram Hoffer, coauthor of the adrenochrome chapter being discussed in this post. It just never became widely used as a treatment for schizophrenia.
Hoffer and two others wrote the book Niacin: The Real Story: Learn about the Wonderful Healing Properties of Niacin, by Abram Hoffer, MD, PhD, Andrew Saul, PhD, and Harold D. Foster, Ph.D. Abram Hoffer found high dose niacin (3 doses of 1000 mg per day) beneficial for patients with schizophrenia - in 1971. (2nd Edition on Amazon)
History of Dr. Hoffer’s use of niacin to treat schizophrenia is here: (mcgill.ca)
Is niacin a possible successful treatment for schizophrenia?
“Schizophrenia is a devastating disease. Characterized by hallucinations, delusions and disorganized thought, it destroys lives. While the condition can be controlled with appropriate medication, a cure remains elusive. But just what constitutes “appropriate medication” is controversial.” […]
“Antipsychotic drugs that block dopamine and serotonin receptors in the brain have been the mainstay of therapy, but the involuntary muscle movements, restlessness and tremors they can cause are troublesome.” Read more: (mcgill.ca)
People with diagnoses of schizophrenia in modern day reportedly have worse quality of life than they did in the early 1900s when sunshine and nutrition were the main treatments. Modern psychiatric drugs have severe negative side effects and don’t really treat the underlying issues that are being discussed here. Or the other nutrient deficiencies that are frequently seen, or a copper transport protein gene difference which can be a genetic cause - the blood might have copper but the brain isn’t getting it delivered. To me, “Schizophrenia” seems more like a symptom of many different potential issues, rather than being a single condition with a single cause (aka: Dx: Lack of psych drugs ;-).
I think the link to adrenochrome chemistry has likely kept this information suppressed, and the use of high dose niacin suppressed from use as a treatment for schizophrenia — or niacin is simply too inexpensive for use in a for-profit medical industry.
Example: $19.99 for 100 tablets of 500 mg Vit. B3, flush niacin, GNC = $1.20 for 6/day - 1000 mg x 3.
*Unaffiliated but I like and have used the product - it is buffered so the acidity is not upsetting to the stomach like pure niacin powder is. If starting a high dose niacin protocol, though, gradually work up from 50 mg or 250 mg and avoid suddenly stopping or forgetting. Serotonin balance is delicately linked to the niacin intake. Gradually increase, and gradually withdraw from high dose niacin to avoid symptoms of serotonin excess while ramping up the dose (physically ill symptoms), or sudden symptoms of serotonin deficiency when stopping use (which might feel like crying or that everyone hates you). (post, 1-2021) (post, 10-2022)
Physiological symptoms of schizophrenia, which might be explained by excess adrenochrome:
Adrenalin oxidizes to Adrenochrome, causing schizophrenia-like thinking. Lack of antioxidants is a risk factor.
Summary issues:
Low niacin, 1000 mg x 3/day has shown clinical benefits;
Antioxidant lack - low endogenous production of glutathione, low cysteine and glycine may be a factor;
low GABA, an amino acid with calming functions as a neurotransmitter in the brain;
Histamine excess;
Phosphorus imbalance, lack of oxidative phosphorylation of ATP;
Copper imbalance or excess of a copper based enzyme, ceruloplasmin; [a genetic flaw in a brain copper transport protein can also be a cause of schizophrenia];
Hypothyroidism, Adrenochrome is antithyroid and 200 mcg iodine supplementation has helped in schizophrenia.
Nutrients can help reduce risk of excess adrenochrome production or accumulation.
Niacin / Nicotinic Acid, Butyrate, (Nicotine)
- lack of B3 may be a metabolic difference. High dose flush niacin - Work up to 1000 mg x 3/day gradually, withdraw slowly too, Serotonin excess or deficiency causes negative symptoms.
- Resistant Starch/fiber & Zinc to support butyrate production in the colon. 25-50 mg zinc/day (>age).
Vit. C, 250 mg/day, caution of Uric Acid excess when using high dose niacin too.
Glutathione support - Cysteine - Glycine (TMG or DMG) - 600 mg cysteine, 3-10 gr glycine, either or both, varied function. Mitochondrial support nutrients are needed too, gene differences may affect which nutrients to supplement or limit.
GABA - amino acid - 200 mg lozenge, dissolve in mouth, as needed for anxiety.
Taurine - Adequate protein / Red meat - 0.5-2 gr taurine / day. May be in energy drinks too, caution if sensitive mentally.
PABA - B vitamin - 1000 mg/day.
Iodine - 200 mcg - 12.5 mg/day (Iodoral).
Selenium - 200 mcg/day or 2 Brazil Nuts.
Omega 3 Fatty Acids, EPA/DHA ~ 1 - 1.5 grams/day
Niacin
The following passage shows adrenochrome is involved with uncoupling of mitochondrial ATP production similar to what high dose niacin can do with activation of the GP109a receptor. *More on niacin, butyrate, and other agonists for the GP109a receptor as potential therapeutics for schizophrenia is here: (Oxenkrug and Forester, 2024), and excerpted after the adrenochrome section
Lack of niacin may be present in people with schizophrenia: This passage explains why people with schizophrenia likely need the 3000 mg of niacin/nicotinic acid each day - they may have “less of the enzyme that converts “3-hydroxyanthranilic acid into nicotinic acid”:
“Ehrensvard et al. (1960) and Heath and Leach (1962) found that schizophrenic serum oxidized 3-hydroxyanthranilic acid enzymatically more slowly than did normal serum. This suggests schizophrenic serum contains less of the enzyme which converts 3-hydroxyanthranilic acid into nicotinic acid. The result would be an increase in 3-hydroxyanthranilic acid, an increase in 3-hydroxykynurenine, an increase in ommochromes formation, and a deficiency of nicotinic acid.”
(page 287, Chapter III. Adrenochrome and Some of Its Derivatives, The Hallucinogens, by A. Hoffer and H. Osmond, 1967, Academic Press, (pdf in my Dropbox)
Reduced glutathione or lack of glycine would add to accumulation of adrenochrome: Lack of glutathione or cysteine or glycine would be likely to increase adrenochrome accumulation too. And excess of it depletes glycine and glutathione. That helps explain my risk in part - genetically low in dimethylglycine, DMG.
Low GABA - less calming neurotransmitter: Excess adrenochrome would then lead to reduced GABA which is calming and that would just add to the mental over-activity.
Histamine excess may be present and a seeming insensitivity to it: Adrenochrome can reduce sensitivity to histamine and schizophrenia patients have been found to have higher levels of histamine than average while not having as quick of a reddening reaction on the skin when scratched.
Dietary food sources could make this worse and a gene difference in two enzymes needed to deactivate histamine could be a risk factor, or reduced methylation function as that is also needed - histamine is methylated to deactivate it.
Retinoid Toxicity would make histamine worse and schizophrenia is associated with both low vitamin A and excess Retinoids - suggesting that a lack of zinc, taurine, and/or adequate protein could be causal factors too.
»> Disoriented thinking can have multiple underlying factors.
High pressure oxygen causes similar disoriented thinking or reckless behavior: High pressure oxygen situations replicate the poor judgement or distorted thinking of schizophrenia and can be seen in ‘the Bends’ after surfacing too quickly after a deep-sea dive. More info on deep sea diving risk of the Bends and reckless behavior see: (Brave AI summary).
Hypothyroidism can become likely, needing generous Synthroid doses, or try iodine!: Adrenochrome excess may cause schizophrenics to get colder in cold temperatures more than average. Adrenochrome excess may add to anti-thyroid problems. Schizophrenia patients seemed to tolerate larger and need larger than average doses of thyroid treatment (pre days of Synthroid - something like porcine thyroid extract was used). Or 200 micrograms of iodine helped patients with schizophrenia. Thyroid function and hormone levels were normal. The problem was found in peripheral use of thyroid hormone. Higher dosing seems to help bypass anti-thyroid effects caused by excess adrenochrome within the body tissues.
There may be excess ceruloplasmin, a copper-based enzyme that converts adrenaline into adrenolutin. That excess ceruloplasmin may be involved in schizophrenia seems protective, like a counteracting effect rather than being the enzyme causing excess adrenochrome conversion:
“Ceruloplasmin, a copper protein enzyme, normally present in serum oxidizes catechol, adrenaline, serotonin, and other amines. Leach et al. (1956) believed it was the catalyst in the oxidation of adrenaline to adrenochrome. Akerfeldt (1957a,b) found that N,N-diethyl-p-phenylenediamine was a useful substrate for measuring ceruloplasmin levels. Akerfeldt reported that schizophrenic patients generally were higher in ceruloplasmin thus supporting the suggestion of Angel et al. (1957) that schizophrenic serum converted more adrenaline into adrenolutin.” (page 315)
“Melander (1957) found that ceruloplasmin absorbs adrenolutin.” (page 316) (Hoffer and Osmond, 1967)
Phosphorus imbalance: Retaining phosphorus, or losing too much - phosphate imbalance also seems involved in schizophrenia genetics - that might go hand in hand with fibromyalgia seeming to have odd phosphorus issues too. Adrenochrome inhibits ATPase enzyme, needed for oxidative phosphorylation. If it isn’t used normally, then the phosphorus can collect in cells.
Dietary background info: an overly acidic body chemistry promotes more blood phosphorus, and lack of calcium and magnesium would add to phosphorus imbalance. Lack of vitamin K2 and vitamin D wouldn’t help either.
Regarding disrupted phosphorus metabolism - it is likely a direct result from the uncoupling of ATP production - the cell isn’t using it as much so it collects instead. Adrenochrome inhibits ATPase, but adrenalin doesn’t do that:
“There is a good deal of data which show that schizophrenic erythrocytes do not metabolize glucose in the same way as normal erythrocytes. Boszormenyi-Nagy and Gerty (1955) found that insulin pretreatment of normal erythrocytes inhibited the accumulation of adenosine triphosphate (ATP) by hemolysates incubated with pyruvate and hexose diphosphate. This inhibition of ATP buildup was not observed with schizophrenic erythrocytes. Since adrenochrome is a very powerful inhibitor of ATPase while adrenaline alone has no effect it is likely this differential effect of erythrocytes is due to an accumulation of oxidized adienaline derivatives. That is with normal erythrocytes insulin would increase utilization of ATP but with ATPase inhibited by adrenochrome this could not occur in schizophrenic cells. Orstrum and Skaug (1950) also found a decreased turnover of ATP in schizophrenics. Randall (1946) and Meyerhoff, and Randall (1948) found that adrenochrome inhibited hexokinase and phosphohexokinase. A concentration of 4 X 10 — 5 M inhibited these enzymes 50% . The degree of inhibition was inversely related to the amount of ATP present.
These earlier findings have some relevance to recent work on toxic protein fractions in schizophrenics. Frohman et al. (1960) found that schizophrenic red cells under basal conditions took up much more labeled 32 P [radioactive labeled phosphorus to track it in lab settings] than did normal subjects. There was an inability to utilize ATP.” (page 307, Hoffer and Osmond, 1967)
Possible benefits of excess adrenochrome genetics
…(besides nice brown hair that doesn’t turn gray early in life ;-)
Reduced risk for diabetes: Adrenochrome excess seems to protect against diabetes that is due to lack of insulin, by inhibiting breakdown of insulin by insulinase.
Anti-aging may be a real benefit from adrenochrome, by reducing cell division or possibly by effects on mitochondrial function.
Adrenochrome is a topic I looked into at the request of someone, and I saw that there had been a bunch of research on it in the 50s and then not much. I learned that it caused ‘schizophrenia-like symptoms’ but might have anti-aging effects due to effects on mitochondrial health.
The alleged anti-aging effect of adrenochrome may be a reduction in cell division - cells survive longer before dividing into two new cells.
“However, we would expect that schizophrenics would have less clear diurnal rhythm of mitosis if they really do have increased concentrations of adrenochrome. Increased quantities of adrenochrome-chalone would suppress mitosis and promote cell survival. Schizophrenics would be expected to have better cell survival rates and, indeed, many schizophrenics do appear to be remarkably youthful in appearance.” (page 317, Hoffer and Osmond, 1967)
Adrenochrome was observed to interfere with tadpole metamorphosis, which suggests to me that excess adrenochrome negatively affects quantum energy flow and structuring of cell water - disrupting the holographic like formation of a new shape for the developing frog. Fetal development is or involves ‘quantum biology,’ whatever that term means these days.
“Rawson et al. (1957) stated that adrenochrome prevented metamorphosis of tadpoles. This is a property shared with other indoles and provides direct evidence that thyroid hormone and adrenochrome are antagonists.
It is curious that both thyroxine and adrenochrome are uncouplers of oxidative phosphorylation. J. Bain (1957) reported that a subeffective dose of thyroxin (5 X 10—5 M) and a subeffective dose of adrenochrome ( 2 X 10—5 M) added together produced 50% uncoupling of oxidative phosphorylation.” (page 303, Hoffer and Osmond, 1967))
“Uncoupling oxidative phosphorylation” is similar to what the GP109a receptor can do when activated - mitochondria can uncouple from ATP production and release energy as warmth instead - like brown adipose tissue. More on the GP109a receptor is at the end of the post.
Potential treatments for patients with schizophrenia:
Inhibitors of Adrenaline oxidase - EDTA and ascorbic acid. Copper chelating agents would inhibit adrenaline oxidase.
Inhibitor of tryosinase - PABA (a B vitamin) inhibits tryosinase from oxidizing adrenaline to adrenochrome. Other inhibitors of the enzyme include “cysteine, thioureas, glycine, and histidine (Hirsch, 1959), and monohydroxybenzoic acid isomers (Yasunobu, 1959),” and Benzoic acid and dPhenylalanine. (p 336)
“Adrenaline Oxidase Inhibitors. Payza and Hoffer (1959) found only 4 inhibitors of this enzyme, sodium cyanide, ethylenediaminetetraacetate (EDTA) tris buffer and ascorbic acid. Sodium cyanide could not be tested as an hallucinogen. In nontoxic doses it has produced lucid episodes in some schizophrenic patients. EDTA has not been used clinically and its toxic properties are not known. We would expect it to be nontoxic and, in fact, therapeutic for schizophrenics as is penicillamine. Both are copper chelating agents and by removing copper would inhibit adrenaline oxidase. G. J. Martin et al. (1942) found that p-aminobenzoic acid inhibited oxidation of adrenaline to adrenochrome by tryosinase. We have not heard of any psychotic reactions following the use of heavy doses of PABA. In its absence convulsions may develop. PABA should be therapeutic for schizophrenia.
Inhibitors of tryosinase also include cysteine, thioureas, glycine, and histidine (Hirsch, 1959), and monohydroxybenzoic acid isomers (Yasunobu, 1959). Benzoic acid itself is a good inhibitor. These compounds may be valuable therapeutic chemicals for some schizophrenics, dPhenylalanine is another inhibitor and is believed responsible for the deficiency of melanization in phenylpyruvic oligophrenia.” (page 336, Hoffer and Osmond, 1967)
Chapter III. Adrenochrome and Some of Its Derivatives, The Hallucinogens, by A. Hoffer and H. Osmond, 1967, Academic Press, (pdf in my Dropbox)
Why is a high dose of niacin needed?
— to reach a great enough concentration to activate the GP109a receptor - it helps reduce inflammation and promotes removal of cellular debris by white blood cells using endolysomes - cellular garbage treatment organelles/cell parts.
I was just reviewing why the ‘high dose’ is needed for the GP109a activation to occur - niacin needs to be present at higher concentrations, at medicinal amounts rather than at dietary levels, for it to act as an agonist of the GP109a receptor. Butyrate may be the more typical agonist of the receptor, and butyrate is critical in colon health for immune support and as an energy source — eat adequate zinc and resistant starches to support butyrate producing species. They make the butyrate out of the resistant and other fibers and need about 30% of our dietary zinc each day.
Agonists of the GP109a receptor discussed by (Oxenkrug and Forester, 2024):
Nicotinic acid is niacin - needed at “pharmacological doses … (4 gr)”. (Oxenkrug and Forester, 2024)
Butyric acid (BA) in the excerpt below is chemically related to butyrate, different by one proton and likely the form used in supplements. Butyrate is the conjugated base of the butyric acid. (biocrates.com/butyric acid)
Beta-hydroxybutyrate (BHB) is associated with anti-aging. Ketosis diets are the known method for increasing our endogenous production of it. (Han, et al., 2020)
Anthranilic acid (AA) “AA is one of the 3 immediate down-stream catabolites of kynurenine (Kyn) formed along the Trp–Kyn–nicotinamide adenine dinucleotide (NAD+) pathway.” (Oxenkrug and Forester, 2024)
“Limited clinical efficiency of current medications warrants search for new antipsychotic agents.
Deorphanized G-protein coupled receptor (GPR)109A has not attracted much of attention of schizophrenia researchers.
We analyzed literature and our data on endogenous agonists of GPR109A, beta-hydroxybutyrate (BHB), anthranilic (AA), butyric (BA), and nicotinic (NA) acids, in individuals with schizophrenia.” via (TPi137 on TG) […]
“- Although NA binds to GPR109a with high affinity (100 nM EC50), this concentration is only reached in response to the administration of pharmacological doses of NA (4 g) while under physiological conditions, NA’s blood levels are too low to activate GPR109A.
Therefore, pharmacological doses of NA are required for both GPR109A activation and the antipsychotic effects.
These findings are in line with the suggestion that activation of GPR109A mediates anti-psychotic effect of NA.
- The human GPR109A gene is located on the long arm of chromosome 12 at position 24.31 (notated as 12q24.31).
- Myelin is a phospholipid sheath around nerve cell axons to protect and promote nerve conduction. Decreased myelination is associated with more rapid cognitive decline among cognitively unimpaired individuals and with cognitive dysfunction in individuals with schizophrenia.
- GPR109A expression has not been assessed in schizophrenia, except genome wide study that suggested an association of genetic mutation of GPR109A with diminished flush in response to NA, a feature of a subgroup of schizophrenia patients. Expression of GPR109A might identify a new endophenotype of schizophrenia." (Oxenkrug and Forester, 2024) via TPi137 (in my Telegram chat)
Adrenalin oxidizes to Adrenochrome, causing schizophrenia-like thinking.
Lack of antioxidants, stress and terror are involved; gene differences can increase risk of adrenochrome excess.
Symptoms of adrenochrome excess:
Disoriented thinking, hallucinations, paranoia, delusions, visual or spatial distortions of perception.
Lack of self-awareness that these differences are internal rather than “the neighbor spying.”
Reckless behavior, no regard for risk to self or others - “dumb mistakes”, car accidents. Similar to the Bends after deep-sea diving, high pressure oxygen causes excess oxidation of adrenalin into adrenochrome.
Nutrients can help reduce risk of excess adrenochrome production.
Niacin / Nicotinic Acid, Butyrate, (Nicotine)
- lack of B3 may be a metabolic difference. High dose flush niacin - Work up to 1000 mg x 3/day gradually, withdraw slowly too, Serotonin excess or deficiency causes negative symptoms.
- Resistant Starch/fiber & Zinc to support butyrate production in the colon. 25-50 mg zinc/day (>age).
Vit. C, 250 mg/day, caution of Uric Acid excess when using high dose niacin too.
Glutathione support - Cysteine - Glycine (TMG or DMG) - 600 mg cysteine, 3-10 gr glycine, either or both, varied function. Mitochondrial support nutrients are needed too, gene differences may affect which nutrients to supplement or limit.
GABA - amino acid - 200 mg lozenge, dissolve in mouth, as needed for anxiety.
Taurine - Adequate protein / Red meat - 0.5-2 gr taurine / day. May be in energy drinks too, caution if sensitive mentally.
PABA - B vitamin - 1000 mg/day.
Iodine - 200 mcg - 12.5 mg/day (Iodoral).
Selenium - 200 mcg/day or 2 Brazil Nuts.
Omega 3 Fatty Acids, EPA/DHA ~ 1 - 1.5 grams/day
Didn’t fit on this graphic - Vitamin D3, 1000-2000 IU - and avoid glyphosate, and vitamin K2, high dose preferred, 45 mg.
Magnesium adequacy, topical Epsom salt is a bioactive sulfate source which can help support sulfation if it is not excessive for sulfation function.
Lifestyle factors that might affect risk of excess adrenochrome.
Stress depletes antioxidants needed to -> adrenalin into safer chemicals than adrenochrome - methylation & sulfation
Reducing stressors of any type would help, along with Epsom salt baths - 20-minute soaks, 1 cup salt, 1-2 x week. If racing heartrate occurs, then sulfur sensitivity may be a problem. Reduce sulfur foods or preservatives in the diet and limit time in an Epsom salt soak. An excess of magnesium from a bath would cause a slowing of heart rate and loose, watery stools for a full day - so don’t stay in longer than 40 minutes as a general caution for anyone. Magnesium and sulfate can be absorbed through our skin pores, hair follicles, and around our finger and toenails.
Avoid flickering lights if histamine sensitive, and anything personally allergic or sensitized to. See page Histamine/MCAS on jenniferdepew.com/index.
Practice good sleep habits - black out darkness, and get early morning and midday sunshine, in order to support circadian cycle function and our mitochondria and gut microbiome. See: Sleep and Health, (denutrients.substack).
Have adequate water, and exercise with full range of motion and some weight bearing exercise. Circuit training with shorter bursts of intense exercise can be beneficial once healthy enough for that level of aerobic intensity.
Reduce EMF exposure as much as possible. Go wired and give up WiFi at home, or turn off the modem at night are some simple steps. An EMF blocking cellphone case can help reduce exposure when it is not in use - and protects against data hacking in public settings.
»> Check out the Substack by Roman S Shapoval and sign up for his next session of EMF 101 with live webinars weekly and pre-recorded material for background info and how to reduce EMF step by step in your home.
LOCATION counts, the laptop on your lap is worse for your health than the cell tower down the street because of the close proximity. Cancers do occur in locations where people wear a cellphone or use a cellphone a lot. Keeping your cellphone in your back pocket or bra is bad for your health.
Dietary Therapeutic Goals.
Methylation Support nutrients - may vary based on individual gene differences. Methyl folate, choline, methyl or hydroxy B12, are needed in addition to B1, B2, B3, B6, biotin, and betaine (TMG) or DMG. Also, antioxidants: Alpha Lipoic Acid & CoQ10, and minerals incl. manganese, magnesium, zinc, & iron. Molybdenum and boron can also help. See this post for more on methylation and sulfation, and molybdenum food sources, also why a gluten free diet can be important for a lot of people with inflammatory or stress/gut issues: (denutrients.substack) See pages Prenatal/Child, Nutrients, or Cofactors, on jenniferdepew.com/index, for more on dosing of various nutrients and other supplements.
Methylation function is needed for our production of Glutathione and supports Mitochondrial function and health. Sulfation helps detox many chemicals.
Gut Microbiome support for Butyrate species - Resistant Starch/fiber & Zinc to support butyrate production in the colon. 25-50 mg zinc/day (>age). Fiber & Resistant Starch are macronutrients - we need salad type servings rather than a capsule. See pages Zinc, Resistant Starch/Butyrate and How much Butyrate? on jenniferdepew.com/index.
Sulfation support -avoid excessive sulfate foods & preservatives as we all process sulfur slowly - it would be even worse if methylation function is poor.
Low Histamine diet and lifestyle - reduce fermented and aged leftovers or aged cheese, canned goods, pickles, tomatoes, walnuts, .... others; flickering or strobe lights, action movies - wear dark sunglasses. Flickering trigger Mast cells, incr. histamine levels. DOA enzyme may help. See: Histamine Food List: (my dropbox.com, pdf) See page Histamine/MCAS on jenniferdepew.com/index.
Alkaline/low acid producing diet - plant foods, less dairy and rich meats. Avoid carbonated beverages. Lemon, lime, apple cider vinegar are beneficial though, mimicking stomach acid and aiding digestion for older adults. See: Alkalizing Foods and other inflammatory food categories - lectins, TRP channel activators, oxalates - (my dropbox.com pdf)
Other Physical Symptoms associated with Schizophrenia or Excess Adrenochrome
Adrenochrome has antithyroid effects, hypothyroidism may occur and cold/hot sensitivity.
Histamine excess, with seeming insensitivity to it may occur. Can cause mania or anxiety & paranoia, hypersexuality, allergy symptoms. Red lines will stay reddened on the skin after being scraped.
Reduced methylation function and poor sulfation may be problems leading to additional symptoms and increase risk for adrenochrome excess.
Risk of adrenochrome excess may increase for post-menopausal women as estrogen may help regulate monoamine oxidase enzyme activity.
Adrenalin is made into something other than adrenochrome when Monoamine oxidase (A), Methyl transferase, and Sulfoesterase (C) work. Methylation & sulfation function are needed. Oxidation -> adrenochrome.
Dietary Therapeutic Goals
Calcium/Phosphorus balance
Vitamin D & K2 are needed to keep calcium in the bones instead of going to blood & soft tissue where it can inhibit MOA enzyme.
Presoak beans, nuts, seeds & grains overnight & rinse before cooking to reduce phytic acid which promotes phosphorus imbalance & acidity.
Sulfation support is important because ammonia inhibits, monoamine oxidase, an enzyme needed to deactivate adrenalin & Metanephrine to something besides adrenochrome. - MOA inhibitors are used as antidepressant medications. Too little MOA activity or too much is associated with Alzheimer’s dementia.
Estrogen can up-regulate MOA activity, shown with hormone replacement therapy in post-menopausal women. Resveratrol & soy genistein may be helpful.
Alkaline/low acid producing diet - lower sugar, fat & simple starches - may be less oxidizing, and have a better calcium/magnesium & phosphorus balance.
Disclaimer: This information is being provided for educational purposes within the guidelines of Fair Use and is not intended to provide individual health care guidance.
Reference List
(Bortolato and Shih, 2011) Bortolato M, Shih JC. Behavioral outcomes of monoamine oxidase deficiency: preclinical and clinical evidence. Int Rev Neurobiol. 2011;100:13-42. doi: 10.1016/B978-0-12-386467-3.00002-9. PMID: 21971001; PMCID: PMC3371272. https://pmc.ncbi.nlm.nih.gov/articles/PMC3371272/
(Cao, et al., 2007) Cao, X., Wei, Z., Gabriel, G.G. et al. Calcium-sensitive regulation of monoamine oxidase-A contributes to the production of peroxyradicals in hippocampal cultures: implications for Alzheimer disease-related pathology. BMC Neurosci 8, 73 (2007). https://doi.org/10.1186/1471-2202-8-73 https://bmcneurosci.biomedcentral.com/articles/10.1186/1471-2202-8-73
(Chakravorty and Halbreich, 1997) Chakravorty SG, Halbreich U. The influence of estrogen on monoamine oxidase activity. Psychopharmacol Bull. 1997;33(2):229-33. PMID: 9230635. https://pubmed.ncbi.nlm.nih.gov/9230635/
(Han, et al., 2020) Han, YM., Ramprasath, T. & Zou, MH. β-hydroxybutyrate and its metabolic effects on age-associated pathology. Exp Mol Med 52, 548–555 (2020). https://doi.org/10.1038/s12276-020-0415-z https://www.nature.com/articles/s12276-020-0415-z
(Hoffer and Osmond, 1967), Chapter III. Adrenochrome and Some of Its Derivatives, The Hallucinogens, by A. Hoffer and H. Osmond, 1967, Academic Press, (pdf in my Dropbox)
(Holschneider, et al., 1998) D.P. Holschneider, T. Kumazawa, K. Chen, J.C. Shih, Tissue-specific effects of estrogen on monoamine oxidase A and B in the rat, Life Sciences, Vol 63, Issue 3, 1998, pp 155-160, ISSN 0024-3205, https://doi.org/10.1016/S0024-3205(98)00255-0. https://www.sciencedirect.com/science/article/pii/S0024320598002550
(mcgill.ca) Alexandra Pires-Ménard, OSS Intern, Is niacin a possible successful treatment for schizophrenia?, March 20, 2017,
(Oxenkrug and Forester, 2024) Oxenkrug G, Forester B. Anthranilic Acid, a GPR109A Agonist, and Schizophrenia. International Journal of Tryptophan Research. 2024;17. doi:10.1177/11786469241239125 https://journals.sagepub.com/doi/full/10.1177/11786469241239125
(Yeung, et al., 2019) Yeung AWK, Georgieva MG, Atanasov AG, Tzvetkov NT. Monoamine Oxidases (MAOs) as Privileged Molecular Targets in Neuroscience: Research Literature Analysis. Front Mol Neurosci. 2019 May 29;12:143. doi: 10.3389/fnmol.2019.00143. PMID: 31191248; PMCID: PMC6549493. https://www.frontiersin.org/journals/molecular-neuroscience/articles/10.3389/fnmol.2019.00143/full











Wow! Some interesting information for sure!!!
I can def see now why the elite monsters running this matrix zoo have such little sympathy and empathy for those they feel are inferior to them!!!
Jennifer.. Excellent article on a controversial topic. Very informative..
If you don't mind, I would like to crosspost it to increase visibility and education on the topic.